Genotypic Evaluation of Cowpea Germplasm for Salinity Tolerance at Germination and During Seedling Growth
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Full Text Article
SJIF Impact Factor: 3.458 WORLD JOURNAL OF ADVANCE ISSN: 2457-0400 Alvine et al. PageVolume: 1 of 3.21 HEALTHCARE RESEARCH Issue: 4. Page N. 07-21 Year: 2019 Original Article www.wjahr.com ASSESSING THE QUALITY OF LIFE IN TOOTHLESS ADULTS IN NDÉ DIVISION (WEST-CAMEROON) Alvine Tchabong1, Anselme Michel Yawat Djogang2,3*, Michael Ashu Agbor1, Serge Honoré Tchoukoua1,2,3, Jean-Paul Sekele Isouradi-Bourley4 and Hubert Ntumba Mulumba4 1School of Pharmacy, Higher Institute of Health Sciences, Université des Montagnes; Bangangté, Cameroon. 2School of Pharmacy, Higher Institute of Health Sciences, Université des Montagnes; Bangangté, Cameroon. 3Laboratory of Microbiology, Université des Montagnes Teaching Hospital; Bangangté, Cameroon. 4Service of Prosthodontics and Orthodontics, Department of Dental Medicine, University of Kinshasa, Kinshasa, Democratic Republic of Congo. Received date: 29 April 2019 Revised date: 19 May 2019 Accepted date: 09 June 2019 *Corresponding author: Anselme Michel Yawat Djogang School of Pharmacy, Higher Institute of Health Sciences, Université des Montagnes; Bangangté, Cameroon ABSTRACT Oral health is essential for the general condition and quality of life. Loss of oral function may be due to tooth loss, which can affect the quality of life of an individual. The aim of our study was to evaluate the quality of life in toothless adults in Ndé division. A total of 1054 edentulous subjects (partial, mixed, total) completed the OHIP-14 questionnaire, used for assessing the quality of life in edentulous patients. Males (63%), were more dominant and the ages of the patients ranged between 18 to 120 years old. Caries (71.6%), were the leading cause of tooth loss followed by poor oral hygiene (63.15%) and the consequence being the loss of aesthetics at 56.6%. -

A Collection of 100 Proverbs and Wise Sayings of the Medumba (Cameroon)
A COLLECTION OF 100 PROVERBS AND WISE SAYINGS OF THE MEDUMBA (CAMEROON) BY CIKURU BISHANGI DEVOTHA AFRICAN PROVERBS WORKING GROUP NAIROBI KENYA MAY 2019 i ACKNOWLEDGEMENT I wish to thank sincerely all those who contributed towards the completion of this document. My greatest thanks go to Fr. Joseph G. Healey for the financial and moral support. My special thanks go to Cephas Agbemenu, Margret Wambere Ireri and Father Joseph Healey. I also thank all the publishers of African Proverbs whose publications gave me good resource and inspiration to do this work. I appreciate the support of the African Proverbs Working Group for reviewing the progress of my work during their meetings. Find in this work all my gratitude. DEDICATION I dedicate this work to God for protecting and strengthening me while working on the Medumba. To HONDI M. Placide, my husband and my mentor who encouraged me to complete this research, To my collaborator Emmanuel Fandja who helped me to collect the 100 Medumba Proverbs. To all the members of my community in Nairobi and to all those who will appreciate in finding out the wisdom that it contains. i A COLLECTION OF 100 MEDUMBA PROVERBS AND WISE SAYINGS (CAMEROOON) INTRODUCTION Location The Bamileke is the native group which is now dominant in Cameroon's West and Northwest Regions. It is part of the Semi-Bantu (or Grassfields Bantu) ethnic group. The Bamileke are regrouped under several groups, each under the guidance of a chief or fon. Nonetheless, all of these groups have the same ancestors and thus share the same history, culture, and languages. -
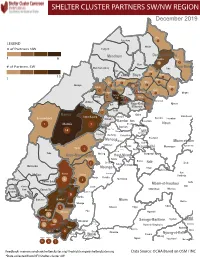
NW SW Presence Map Complete Copy
SHELTER CLUSTER PARTNERS SW/NWMap creation da tREGIONe: 06/12/2018 December 2019 Ako Furu-Awa 1 LEGEND Misaje # of Partners NW Fungom Menchum Donga-Mantung 1 6 Nkambe Nwa 3 1 Bum # of Partners SW Menchum-Valley Ndu Mayo-Banyo Wum Noni 1 Fundong Nkum 15 Boyo 1 1 Njinikom Kumbo Oku 1 Bafut 1 Belo Akwaya 1 3 1 Njikwa Bui Mbven 1 2 Mezam 2 Jakiri Mbengwi Babessi 1 Magba Bamenda Tubah 2 2 Bamenda Ndop Momo 6b 3 4 2 3 Bangourain Widikum Ngie Bamenda Bali 1 Ngo-Ketunjia Njimom Balikumbat Batibo Santa 2 Manyu Galim Upper Bayang Babadjou Malentouen Eyumodjock Wabane Koutaba Foumban Bambo7 tos Kouoptamo 1 Mamfe 7 Lebialem M ouda Noun Batcham Bafoussam Alou Fongo-Tongo 2e 14 Nkong-Ni BafouMssamif 1eir Fontem Dschang Penka-Michel Bamendjou Poumougne Foumbot MenouaFokoué Mbam-et-Kim Baham Djebem Santchou Bandja Batié Massangam Ngambé-Tikar Nguti Koung-Khi 1 Banka Bangou Kekem Toko Kupe-Manenguba Melong Haut-Nkam Bangangté Bafang Bana Bangem Banwa Bazou Baré-Bakem Ndé 1 Bakou Deuk Mundemba Nord-Makombé Moungo Tonga Makénéné Konye Nkongsamba 1er Kon Ndian Tombel Yambetta Manjo Nlonako Isangele 5 1 Nkondjock Dikome Balue Bafia Kumba Mbam-et-Inoubou Kombo Loum Kiiki Kombo Itindi Ekondo Titi Ndikiniméki Nitoukou Abedimo Meme Njombé-Penja 9 Mombo Idabato Bamusso Kumba 1 Nkam Bokito Kumba Mbanga 1 Yabassi Yingui Ndom Mbonge Muyuka Fiko Ngambé 6 Nyanon Lekié West-Coast Sanaga-Maritime Monatélé 5 Fako Dibombari Douala 55 Buea 5e Massock-Songloulou Evodoula Tiko Nguibassal Limbe1 Douala 4e Edéa 2e Okola Limbe 2 6 Douala Dibamba Limbe 3 Douala 6e Wou3rei Pouma Nyong-et-Kellé Douala 6e Dibang Limbe 1 Limbe 2 Limbe 3 Dizangué Ngwei Ngog-Mapubi Matomb Lobo 13 54 1 Feedback: [email protected]/ [email protected] Data Source: OCHA Based on OSM / INC *Data collected from NFI/Shelter cluster 4W. -

Skeeling 1.Pdf
Musicking Tradition in Place: Participation, Values, and Banks in Bamiléké Territory by Simon Robert Jo-Keeling A dissertation submitted in partial fulfillment of the requirements for the degree of Doctor of Philosophy (Anthropology) in The University of Michigan 2011 Doctoral Committee: Professor Judith T. Irvine, Chair Emeritus Professor Judith O. Becker Professor Bruce Mannheim Associate Professor Kelly M. Askew © Simon Robert Jo-Keeling, 2011 acknowledgements Most of all, my thanks go to those residents of Cameroon who assisted with or parti- cipated in my research, especially Theophile Ematchoua, Theophile Issola Missé, Moise Kamndjo, Valerie Kamta, Majolie Kwamu Wandji, Josiane Mbakob, Georges Ngandjou, Antoine Ngoyou Tchouta, Francois Nkwilang, Epiphanie Nya, Basil, Brenda, Elizabeth, Julienne, Majolie, Moise, Pierre, Raisa, Rita, Tresor, Yonga, Le Comité d’Etudes et de la Production des Oeuvres Mèdûmbà and the real-life Association de Benskin and Associa- tion de Mangambeu. Most of all Cameroonians, I thank Emanuel Kamadjou, Alain Kamtchoua, Jules Tankeu and Elise, and Joseph Wansi Eyoumbi. I am grateful to the Wenner-Gren Foundation for Anthropological Research for fund- ing my field work. For support, guidance, inspiration, encouragement, and mentoring, I thank the mem- bers of my dissertation committee, Kelly Askew, Judith Becker, Judith Irvine, and Bruce Mannheim. The three members from the anthropology department supported me the whole way through my graduate training. I am especially grateful to my superb advisor, Judith Irvine, who worked very closely and skillfully with me, particularly during field work and writing up. Other people affiliated with the department of anthropology at the University of Michigan were especially helpful or supportive in a variety of ways. -

Forets Sacres Au Cameroun.Pdf
M.E.M Millennium Ecologic Museum Ministère des Forêts et de la Faune Inventaire, cartographie et étude diagnostic des forêts sacrées du Cameroun : contribution à l’élaboration d’une stratégie nationale de gestion durable RAPPORT FINAL D’EXECUTION Avec l’appui financier du Programme CARPE-IUCN Juin 2010 0 Millennium Ecologic Museum MEM BP : 8038 Yaoundé, Cameroun Tel : (237) 99 99 54 08 / 96 68 11 34 e-mail : [email protected] Site web: www.ecologicalmuseum.netsons.org 1 L’EQUIPE DU PROJET Conseiller Technique Principal Prof. Bernard-Aloys NKONGMENECK, Directeur du MEM Point Focal MINFOF M. NOBANZA Francis, MINFOF/SDIAF - Service Cartographie Conseillers Scientifiques Guy Merlin NGUENANG Vincent BELIGNE Coordination des activités terrain et rapportage Evariste FONGNZOSSIE Collecte des données Victor Aimé KEMEUZE et René Bernadin JIOFACK Assistés de : JOHNSON Madeleine MAKEMTEU Junelle KAMDEM Gyslène WONKAM Christelle TABI Paule Pamela TAJEUKEM Vice Clotex MVETUMBO Moise KENNE Florette Base de Données et GIS Victor KEMEUZE et Francis NOBANZA 2 SOMMAIRE DU RAPPORT LISTE DES ACRONYMES....................................................................................................................................... 6 LISTE DES FIGURES .............................................................................................................................................. 7 LISTE DES TABLEAUX ......................................................................................................................................... -

Commune De Bazou
REPUBLIQUE DU CAMEROUN REGION DE L’OUEST DEPARTEMENT DU NDE COMMUNE DE BAZOU RESERVE FORESTIERE DE BALENGOU RAPPORT D’ENQUÊTE SOCIO-ECONOMIQUE DES VILLAGES RIVERAINS A LA RESERVE FORESTIERE (Katio, Djeufock et Djeuta) JUILLET 2013 Centre Technique de la Forêt Communale Association des Communes Forestières du Cameroun BP 15 107 Yaoundé CAMEROUN Tél. : (00237) 22 20 35 12 Email : ctfccameroun@ yahoo.com Site web : www.foretcommunale-cameroun.org SOMMAIRE Liste des tableaux..............................................................................................................................................4 Liste des cartes..................................................................................................................................................5 Carte 1 Localisation de la commune de Bazou.................................................................................................5 Carte 2 : Carte de localisation des villages riverains à la réserve forestière .................................................5 Carte 3 : Carte de localisation de la réserve forestière dans la commune de Bazou ....................................5 Liste des photos ...................................................................................................................................................5 CHAPITRE 1 : INTRODUCTION ...................................................................................................................6 1.1 Contexte et justification ........................................................................................................................6 -

II. CLIMATIC HIGHLIGHTS for the PERIOD 21St to 30Th JANUARY, 2020
OBSERVATOIRE NATIONAL SUR Dekadal Bulletin from 21st to 30th January, 2020 LES CHANGEMENTS CLIMATIQUES Bulletin no 33 NATIONAL OBSERVATORY ON CLIMATE CHANGE DIRECTION GENERALE - DIRECTORATE GENERAL ONACC ONACC-NOCC www.onacc.cm; email: [email protected]; Tel (237) 693 370 504 CLIMATE ALERTS AND PROBABLE IMPACTS FOR THE PERIOD 21st to 30th JANUARY, 2020 Supervision NB: It should be noted that this forecast is Prof. Dr. Eng. AMOUGOU Joseph Armathé, Director, National Observatory on Climate Change developed using spatial data from: (ONACC) and Lecturer in the Department of Geography at the University of Yaounde I, Cameroon. - the International Institute for Climate and Ing. FORGHAB Patrick MBOMBA, Deputy Director, National Observatory on Climate Change Society (IRI) of Columbia University, USA; (ONACC). - the National Oceanic and Atmospheric ProductionTeam (ONACC) Administration (NOAA), USA; Prof. Dr. Eng. AMOUGOU Joseph Armathé, Director, ONACC and Lecturer in the Department of Geography at the University of Yaounde I, Cameroon. - AccuWeather (American Institution specialized in meteorological forecasts), USA; Eng . FORGHAB Patrick MBOMBA, Deputy Director, ONACC. BATHA Romain Armand Soleil, Technical staff, ONACC. - the African Centre for Applied Meteorology ZOUH TEM Isabella, MSc in GIS-Environment. for Development (ACMAD). NDJELA MBEIH Gaston Evarice, M.Sc. in Economics and Environmental Management. - Spatial data for Atlantic Ocean Surface MEYONG René Ramsès, M.Sc. in Climatology/Biogeography. Temperature (OST) as well as the intensity of ANYE Victorine Ambo, Administrative staff, ONACC the El-Niño episodes in the Pacific. ELONG Julien Aymar, M.Sc. Business and Environmental law. - ONACC’s research works. I. INTRODUCTION This ten-day alert bulletin n°33 reveals the historical climatic conditions from 1979 to 2018 and climate forecasts developed for the five Agro-ecological zones for the period January 21 to 30, 2020. -

Proceedingsnord of the GENERAL CONFERENCE of LOCAL COUNCILS
REPUBLIC OF CAMEROON REPUBLIQUE DU CAMEROUN Peace - Work - Fatherland Paix - Travail - Patrie ------------------------- ------------------------- MINISTRY OF DECENTRALIZATION MINISTERE DE LA DECENTRALISATION AND LOCAL DEVELOPMENT ET DU DEVELOPPEMENT LOCAL Extrême PROCEEDINGSNord OF THE GENERAL CONFERENCE OF LOCAL COUNCILS Nord Theme: Deepening Decentralization: A New Face for Local Councils in Cameroon Adamaoua Nord-Ouest Yaounde Conference Centre, 6 and 7 February 2019 Sud- Ouest Ouest Centre Littoral Est Sud Published in July 2019 For any information on the General Conference on Local Councils - 2019 edition - or to obtain copies of this publication, please contact: Ministry of Decentralization and Local Development (MINDDEVEL) Website: www.minddevel.gov.cm Facebook: Ministère-de-la-Décentralisation-et-du-Développement-Local Twitter: @minddevelcamer.1 Reviewed by: MINDDEVEL/PRADEC-GIZ These proceedings have been published with the assistance of the German Federal Ministry for Economic Cooperation and Development (BMZ) through the Deutsche Gesellschaft für internationale Zusammenarbeit (GIZ) GmbH in the framework of the Support programme for municipal development (PROMUD). GIZ does not necessarily share the opinions expressed in this publication. The Ministry of Decentralisation and Local Development (MINDDEVEL) is fully responsible for this content. Contents Contents Foreword ..............................................................................................................................................................................5 -

CAMEROON, YEAR 2020: Update on Incidents According to the Armed Conflict Location & Event Data Project (ACLED) Compiled by ACCORD, 23 March 2021
CAMEROON, YEAR 2020: Update on incidents according to the Armed Conflict Location & Event Data Project (ACLED) compiled by ACCORD, 23 March 2021 Number of reported incidents with at least one fatality Number of reported fatalities National borders: GADM, 6 May 2018b; administrative divisions: GADM, 6 May 2018a; incid- ent data: ACLED, 12 March 2021; coastlines and inland waters: Smith and Wessel, 1 May 2015 CAMEROON, YEAR 2020: UPDATE ON INCIDENTS ACCORDING TO THE ARMED CONFLICT LOCATION & EVENT DATA PROJECT (ACLED) COMPILED BY ACCORD, 23 MARCH 2021 Contents Conflict incidents by category Number of Number of reported fatalities 1 Number of Number of Category incidents with at incidents fatalities Number of reported incidents with at least one fatality 1 least one fatality Violence against civilians 572 313 669 Conflict incidents by category 2 Battles 386 198 818 Development of conflict incidents from 2012 to 2020 2 Strategic developments 204 1 1 Protests 131 2 2 Methodology 3 Riots 63 28 38 Conflict incidents per province 4 Explosions / Remote 43 14 62 violence Localization of conflict incidents 4 Total 1399 556 1590 Disclaimer 5 This table is based on data from ACLED (datasets used: ACLED, 12 March 2021). Development of conflict incidents from 2012 to 2020 This graph is based on data from ACLED (datasets used: ACLED, 12 March 2021). 2 CAMEROON, YEAR 2020: UPDATE ON INCIDENTS ACCORDING TO THE ARMED CONFLICT LOCATION & EVENT DATA PROJECT (ACLED) COMPILED BY ACCORD, 23 MARCH 2021 Methodology on what level of detail is reported. Thus, towns may represent the wider region in which an incident occured, or the provincial capital may be used if only the province The data used in this report was collected by the Armed Conflict Location & Event is known. -

Programmation De La Passation Et De L'exécution Des Marchés Publics
PROGRAMMATION DE LA PASSATION ET DE L’EXÉCUTION DES MARCHÉS PUBLICS EXERCICE 2021 JOURNAUX DE PROGRAMMATION DES MARCHÉS DES SERVICES DÉCONCENTRÉS ET DES COLLECTIVITÉS TERRITORIALES DÉCENTRALISÉES RÉGION DE L’OUEST EXERCICE 2021 SYNTHESE DES DONNEES SUR LA BASE DES INFORMATIONS RECUEILLIES Nbre de Montant des N° Désignation des MO/MOD N° Page Marchés Marchés 1 Services déconcentrés régionaux 14 526 746 000 3 2 Communauté Urbaine de Bafoussam 18 9 930 282 169 5 Département des Bamboutos 3 Services déconcentrés 6 177 000 000 7 4 Commune de Babadjou 12 350 710 000 7 5 Commune de Batcham 8 250 050 004 9 6 Commune de Galim 6 240 050 000 10 7 Commune de Mbouda 25 919 600 000 10 TOTAL 57 1 937 410 004 Département du Haut Nkam 8 Services Déconcentrés 4 81 000 000 13 9 Commune de Bafang 7 236 000 000 13 10 Commune de Bakou 11 146 250 000 14 11 Commune de Bana 6 172 592 696 15 12 Commune de Bandja 14 294 370 000 16 13 Commune de Banka 14 409 710 012 17 14 Commune de Banwa 10 155 249 999 19 15 Commune de Kékem 5 152 069 520 20 TOTAL 71 1 647 242 227 Département des Hauts Plateaux 16 Services déconcentrés départementaux 1 10 000 000 21 17 Commune de Baham 11 195 550 000 21 18 Commune de Bamendjou 12 367 102 880 22 19 Commune de Bangou 20 371 710 000 24 20 Commune de Batié 6 146 050 002 26 TOTAL 50 1 090 412 882 Département du Koung Khi 21 Services Déconcentrés 2 122 000 000 27 22 Commune de Bayangam 6 257 710 000 27 23 Commune de Dembeng 5 180 157 780 28 24 Commune de Pete Bandjoun 12 287 365 000 28 TOTAL 25 847 232 780 Département de la Menoua 25 -
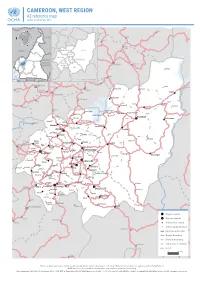
CAMEROON, WEST REGION A3 Reference Map Update of September 2018
CAMEROON, WEST REGION A3 reference map Update of September 2018 Nwa Ndu Benakuma CHAD WUM Nkor Tatum NIGERIA BAMBOUTOS NOUN FUNDONGMIFI MENOUA Elak NKOUNG-KHI CENTRAL H.-P. Njinikom AFRICAN HAUT- KUMBO Mbiame REPUBLIC -NKAM Belo NDÉ Manda Njikwa EQ. Bafut Jakiri GUINEA H.-P. : HAUTS--PLATEAUX GABON CONGO MBENGWI Babessi Nkwen Koula Koutoukpi Mabouo NDOP Andek Mankon Magba BAMENDA Bangourain Balikumbat Bali Foyet Manki II Bangambi Mahoua Batibo Santa Njimom Menfoung Koumengba Koupa Matapit Bamenyam Kouhouat Ngon Njitapon Kourom Kombou FOUMBAN Mévobo Malantouen Balepo Bamendjing Wabane Bagam Babadjou Galim Bati Bafemgha Kouoptamo Bamesso MBOUDA Koutaba Nzindong Batcham Banefo Bangang Bapi Matoufa Alou Fongo- Mancha Baleng -Tongo Bamougoum Foumbot FONTEM Bafou Nkong- Fongo- -Zem -Ndeng Penka- Bansoa BAFOUSSAM -Michel DSCHANG Momo Fotetsa Malânden Tessé Fossang Massangam Batchoum Bamendjou Fondonéra Fokoué BANDJOUN BAHAM Fombap Fomopéa Demdeng Singam Ngwatta Mokot Batié Bayangam Santchou Balé Fondanti Bandja Bangang Fokam Bamengui Mboébo Bangou Ndounko Baboate Balambo Balembo Banka Bamena Maloung Bana Melong Kekem Bapoungué BAFANG BANGANGTÉ Bankondji Batcha Mayakoue Banwa Bakou Bakong Fondjanti Bassamba Komako Koba Bazou Baré Boutcha- Fopwanga Bandounga -Fongam Magna NKONGSAMBA Ndobian Tonga Deuk Region capital Ebone Division capital Nkondjock Manjo Subdivision capital Other populated place Ndikiniméki InternationalBAF borderIA Region boundary DivisionKiiki boundary Nitoukou Subdivision boundary Road Ombessa Bokito Yingui The boundaries and names shown and the designations used on this map do not imply official endorsement or acceptance by the United Nations. NOTE: In places, the subdivision boundaries may suffer of significant inacurracy. Date of update: 23/09/2018 ● Sources: NGA, OSM, WFP ● Projection: WGS84 Web Mercator ● Scale: 1 / 650 000 (on A3) ● Availlable online on www.humanitarianresponse.info ● www.ocha.un.org. -

Bamileke Businessmen in the Realm of Political Transition in Bamileke Region of Cameroon, 1990- 2000
International Journal of Research and Innovation in Social Science (IJRISS) |Volume III, Issue II, February 2019|ISSN 2454-6186 Bamileke Businessmen in the Realm of Political Transition in Bamileke Region of Cameroon, 1990- 2000 Nzeucheu Pascal1, Prof. Simon Tata Ngenge2 1History Department, Faculty of Arts, The University of Bamenda, Cameroon 2Vice Dean, Faculty of Law and Political Science, The University of Bamenda, Cameroon Abstract:-In the Bamilike County of Cameroon the businessmen the party in power, CPDM or for the newly created parties prior to the 1990s were not interested in party politics. After andto obtain representative positions.2 In this perspective, independence they were not interested in politics and business and politic became inseparable. Hence, how the concentrated in building wealth. The creation of a monolithic political transition occurred in the Bamileke area? What were systemon 1st September 1966, made it that they were simple the professional and political identifications of the Bamileke militants of the political system and went about doing their businesses successfully. The re-emergence of multi-party Businessmen that stepped in politics during this period? How democracy in 1990 changed the perception of the businessmen and why businessmen participated in local electoral toward political participation. To protect their businesses most of competitions? Finally what was the impact of this them became militants of Cameroon Democratic Movement participation on the reconfiguration of the Bamileke society? (CPDM) in their various home towns in order to preserve their These are some issues raised in the course of this paper. businesses while other defected from CPDM to join the opposition or created their own political parties.