United States National Museum Bulletin259
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Download This PDF File
Last Name of Authors Examples: Smith Smith & Meyer Smith et al. GUIDELINES FOR MANUSCRIPT PREPARATION Journal Scope. Speleobiology Notes specializes on brief observations on the natural history of subterranean organisms, such as predation events, episodes of reproduction, occurrence in unusual habitats or microhabitats, localized population extinctions, range extensions, new records of species at a given location, and many other natural history phenomena. The journal aims to serve as the primary outlet for much interesting and important information on subterranean fauna contained in the field notes of many speleobiologists, information deemed too fragmented, too brief, too basic, or simply too irrelevant to be included within full-length, traditional scientific journal articles. General Formatting. Manuscripts must be in English. If English is not your native language, please have your manuscript reviewed by a native English-speaking person(s). Manuscripts should be limited to 2500 words or less (not including title, authors and affiliations, key words, and literature cited). The manuscript file should be saved in the native format of the word processing software used (please save as .doc or .docx). To avoid unnecessary errors, you are strongly encouraged to use the ʻspell- checkʼ and ʻgrammar-checkʼ features of your word processing software. The text should be in single-column format. The manuscript should be single-spaced with 2.54 cm (1 inch) margins and 12-point Helvetica font (unless noted otherwise below). For review purposes, please include consecutive page numbers in the page footer and use continuous line numbering throughout the document. Present tables and figure legends on separate pages at the end of the manuscript. -

Mountains, Streams, and Lakes of Oklahoma I
Information Series #1, June 1998 Mountains, Streams, and Lakes of OklahomaI Kenneth S. Johnson2 INTRODUCTION valleys, hills, and plains throughout most of the re mainder of Oklahoma (Fig. 1). All the major lakes and Mountains and streams define the landscape of reservoirs of Oklahoma are man-made, and they are Oklahoma (Fig. 1). The mountains consist mainly of important for flood contr()l, water supply, recreation, resistant rock masses that were folded, faulted, and and generation of hydroelectric power. Natural lakes thrust upward in the geologic past (Fig. 2), whereas in Oklahoma are limited to oxbow lakes along major the streams have persisted in eroding less-resistant streams and to playa lakes in the High Plains region rock units and lowering the landscape to form broad of the west. Alphabetical List of20 Lakes with Largest Surface Area (from Oklahoma Water Atlas, Oklahoma Water Resources Board) 1. Broken Bow 11. Lake 0' The Cherokees 2. Canton 12. Oologah 3. Eufaula 13. Robert s. Kerr 4. Fort Gibson 14. Sardis 5. Foss 15. Skiatook 6. Great Salt Plains 16. Tenkiller Ferry ·7. Hudson 17. Texoma 8. Hugo 18. Waurika 9. Kaw 19. Webbers Falls 10. Keystone 20. Wister Modified from Historical Atlas of Oklahoma, by John W. Morris, Charles R. Goins, and Edwin C. 25 McReynolds. Copyright © 1986 by the University I of Oklahoma Press. o 40 80Km Figure 1. Mountains, streams, and principal lakes of Oklahoma. lReprinted from Oklahoma Geology Notes (1993), vol. 53, no. 5, p. 180-188. The Notes article was reprinted and expanded from Oklahoma Almanac, 1993-1994, Oklahoma Department of Lihraries, p. -

Allocrangonyx Hubrichti)
Conservation Assessment for Hubricht’s Long-Tailed Amphipod (Allocrangonyx Hubrichti) (From Gardner, 1986) USDA Forest Service, Eastern Region October 2002 Julian J. Lewis, Ph.D. J. Lewis & Associates, Biological Consulting 217 W. Carter Avenue Clarksville, IN 47129 [email protected] MARK TWAIN NATIONAL FOREST This Conservation Assessment was prepared to compile the published and unpublished information on Allocrangonyx hubrichti. It does not represent a management decision by the U.S. Forest Service. Though the best scientific information available was used and subject experts were consulted in preparation of this document, it is expected that new information will arise. In the spirit of continuous learning and adaptive management, if you have information that will assist in conserving the subject community and associated taxa, please contact the Eastern Region of the Forest Service Threatened and Endangered Species Program at 310 Wisconsin Avenue, Milwaukee, Wisconsin 53203. Conservation Assessment for Hubricht’s Long-Tailed Amphipod (Allocrangonyx Hubrichti) 2 Table of Contents EXECUTIVE SUMMARY.....................................................................4 NOMENCLATURE AND TAXONOMY...............................................4 DESCRIPTION OF SPECIES................................................................4 LIFE HISTORY......................................................................................4 HABITAT ...............................................................................................5 DISTRIBUTION -

Lazare Botosaneanu ‘Naturalist’ 61 Doi: 10.3897/Subtbiol.10.4760
Subterranean Biology 10: 61-73, 2012 (2013) Lazare Botosaneanu ‘Naturalist’ 61 doi: 10.3897/subtbiol.10.4760 Lazare Botosaneanu ‘Naturalist’ 1927 – 2012 demic training shortly after the Second World War at the Faculty of Biology of the University of Bucharest, the same city where he was born and raised. At a young age he had already showed interest in Zoology. He wrote his first publication –about a new caddisfly species– at the age of 20. As Botosaneanu himself wanted to remark, the prominent Romanian zoologist and man of culture Constantin Motaş had great influence on him. A small portrait of Motaş was one of the few objects adorning his ascetic office in the Amsterdam Museum. Later on, the geneticist and evolutionary biologist Theodosius Dobzhansky and the evolutionary biologist Ernst Mayr greatly influenced his thinking. In 1956, he was appoint- ed as a senior researcher at the Institute of Speleology belonging to the Rumanian Academy of Sciences. Lazare Botosaneanu began his career as an entomologist, and in particular he studied Trichoptera. Until the end of his life he would remain studying this group of insects and most of his publications are dedicated to the Trichoptera and their environment. His colleague and friend Prof. Mar- cos Gonzalez, of University of Santiago de Compostella (Spain) recently described his contribution to Entomolo- gy in an obituary published in the Trichoptera newsletter2 Lazare Botosaneanu’s first contribution to the study of Subterranean Biology took place in 1954, when he co-authored with the Romanian carcinologist Adriana Damian-Georgescu a paper on animals discovered in the drinking water conduits of the city of Bucharest. -
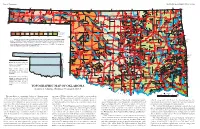
TOPOGRAPHIC MAP of OKLAHOMA Kenneth S
Page 2, Topographic EDUCATIONAL PUBLICATION 9: 2008 Contour lines (in feet) are generalized from U.S. Geological Survey topographic maps (scale, 1:250,000). Principal meridians and base lines (dotted black lines) are references for subdividing land into sections, townships, and ranges. Spot elevations ( feet) are given for select geographic features from detailed topographic maps (scale, 1:24,000). The geographic center of Oklahoma is just north of Oklahoma City. Dimensions of Oklahoma Distances: shown in miles (and kilometers), calculated by Myers and Vosburg (1964). Area: 69,919 square miles (181,090 square kilometers), or 44,748,000 acres (18,109,000 hectares). Geographic Center of Okla- homa: the point, just north of Oklahoma City, where you could “balance” the State, if it were completely flat (see topographic map). TOPOGRAPHIC MAP OF OKLAHOMA Kenneth S. Johnson, Oklahoma Geological Survey This map shows the topographic features of Oklahoma using tain ranges (Wichita, Arbuckle, and Ouachita) occur in southern contour lines, or lines of equal elevation above sea level. The high- Oklahoma, although mountainous and hilly areas exist in other parts est elevation (4,973 ft) in Oklahoma is on Black Mesa, in the north- of the State. The map on page 8 shows the geomorphic provinces The Ouachita (pronounced “Wa-she-tah”) Mountains in south- 2,568 ft, rising about 2,000 ft above the surrounding plains. The west corner of the Panhandle; the lowest elevation (287 ft) is where of Oklahoma and describes many of the geographic features men- eastern Oklahoma and western Arkansas is a curved belt of forested largest mountainous area in the region is the Sans Bois Mountains, Little River flows into Arkansas, near the southeast corner of the tioned below. -

A Checklist and Annotated Bibliography of the Subterranean Aquatic Fauna of Texas
A CHECKLIST AND ANNOTATED BIBLIOGRAPHY OF THE SUBTERRANEAN AQUATIC FAUNA OF TEXAS JAMES R. REDDELL and ROBERT W. MITCHELL Texas Technological College WATER RESOURCES \ CENTER Lubbock, Texas WRC 69-6 INTERNATIONAL CENTER for ARID and August 1969 SEMI-ARID LAND STUDIES A CHECKLIST AND ANNOTATED BIBLIOGRAPHY OF THE SUBTERRANEAN AQUATIC FAUNA OF TEXAS James R. Reddell and Robert W. Mitchell Department of Biology Texas Tech University Lubbock, Texas INTRODUCTION In view of the ever-increasing interest in all studies relating to the water resources of Texas, we have found it timely to prepare this guide to the fauna and biological literature of our subterranean waters. The value of such a guide has already been demonstrated by Clark (1966) in his "Publications, Personnel, and Government Organizations Related to the Limnology, Aquatic Biology and Ichthyology of the Inland Waters of Texas". This publication dea ls primarily with inland surface waters, however, barely touching upon the now rather extensive literature which has accumulated on the biology of our subterranean waters. To state a n obvious fact, it is imperative that our underground waters receive the attention due them. They are one of our most important resources. Those subterranean waters for which biological data exi st are very un equally distributed in the state. The best known are those which are acces sible to collection and study via the entrances of caves. Even in cavernous regions there exist inaccessible deep aquifers which have yielded little in formation as yet. Biological data from the underground waters of non-cave rn ous areas are virtually non-existant. -

Four Western Cheilanthoid Ferns in Oklahoma
Oklahoma Native Plant Record 65 Volume 10, December 2010 FOUR WESTERN CHEILANTHOID FERNS IN OKLAHOMA Bruce A. Smith McLoud High School McLoud, Oklahoma 74851 Keywords: arid, distribution, habitat, key ABSTRACT The diversity of ferns in some of the more arid climates of western Oklahoma is surprising. This article examines four Oklahoma cheilanthoid ferns: Astrolepis integerrima, Cheilanthes wootonii, Notholaena standleyi, and Pellaea wrightiana. With the exceptions of A. integerrima and P. wrightiana which occur in Alabama and North Carolina respectively, all four species reach their eastern limits of distribution in Oklahoma. Included in this article are common names, synonyms, brief descriptions, distinguishing characteristics, U.S. and Oklahoma distribution, habitat information, state abundance, and a dichotomous key to selected cheilanthoids. The Oklahoma Natural Heritage Inventory has determined that all but one (N. standleyi) are species of concern in the state. INTRODUCTION of eastern Oklahoma, while most members of the Pteridaceae occur in Almost half of the ferns in the family western Oklahoma (Taylor & Taylor Pteridaceae are xeric adapted ferns. In 1991). Oklahoma six genera and sixteen species Statewide, the most common species in the family are known to occur. They in the Pteridaceae is Pellaea atropurpurea live on dry or moist rocks and can be (Figure 4), which can be found found in rock crevices, at the bases of throughout the body of the state and boulders, or on rocky ledges. Common Cimarron County in the panhandle. The associated species include lichens, mosses, rarest are Cheilanthes horridula and liverworts, and spike mosses. Two Cheilanthes lindheimeri. Cheilanthes horridula physical characteristics that unite the and Cheilanthes lindheimeri have only been family are the marginal sori (Figure 1) and seen in one county each, Murray and the lack of a true indusium. -

Description of the Coalgate Quadrangle
' *' DESCRIPTION OF THE COALGATE QUADRANGLE. By Joseph A. Taff. GEOGRAPHY. forms of the Ouachita Mountain Range. The a long time the valleys become wide and silted, that at ordinary conditions the stream meanders ridges of the valley region are nearly parallel and the hills are gradually reduced to the level in rivulets or narrow channels. Indeed, its chan GENERAL RELATIONS. with those of the range, but, with the exception of the valleys. nel is so choked with sand that the water does The Coalgate quadrangle is bounded by the of the few isolated mountains which lie in Arkan The surface of the Coalgate quadrangle is of not at any stage of the river flow on the country meridians 96° and 96° 30' and the parallels 34° sas Valley, they have low relief. low relief, and the topographic features indicate rock. During floods, which usually come in 30' and 35°, and thus occupies one- Extentand The Prairie Plains region stretches from the that it has been so for a relatively long period of spring from the headwaters of the river, vast quarter of a square degree of the earth's th1?Juad=f Arkansas Valley and Ozark highland regions time. The larger streams have nearly ceased cut quantities of sands are swept down, shifting for surface. It is 34.4 miles long north rangle> northward and westward across north- Character ting their valleys deeper, and throughout most of mer shoals and channels. Little River, which is and south and 28.5 miles wide, and contains west Indian Territory into Oklahoma their courses are meandering in the deposits of silt tributary to the Canadian, crosses the northwest nearly 980 square miles. -

Distribution of Three Intertidal Cirolanid Isopods (Flabellifera : Cirolanidae) on a South African Sandy Beach
Cah. Biol. Mar. (1992), 33: 147- 168 Roscoff Distribution of three intertidal cirolanid isopods (Flabellifera : Cirolanidae) on a South African sandy beach. A.M.C. De Ruyck, T.E. Donn, jr and A. McLachlan Zoology Department and Institute for Coastal Research, University of Port Elizabeth, P.O. Box 1600, Port Elizabeth, 6000, South Africa Ab5tract : The vertical zonation pattern of Eurydice IOllgicomis. P011loge/oides lalipcs and E.rciro/ana llata/ellSis, cirolanid isopods co-existing on Sundays River Beach, was studied, and differed l'rom Dahl 's, Salvat's and Trevallion Cl al.'s schemes. In view of the apparent differences in zonation both in the present study and the litera ture, the use of cirolanid isopods as indicators of zones is questionable. Environmental factors are suspected to be more important th an biological interactions as cause for the observed zonation patterns of the three species. This study suggests longshore distributional differences in the cirolanid iso pod populations bQth on a large scale along a log-spiral bay and on a 5mall scale along a mega-cusp system. Seasonal changes ln distribution seem to occur in ail three species as weil as semilunar zonation changes in E. l/CIta/cllS is. The t1exible zonation patterns exhibited by the cirolanid isopods at Sundays River Beach may be an adaptation for survival in the dynamic, unstable conditions of a high energy, microtidal beach. Résumé: L'arrangement de la zonation verticale des isopodes cirolanides coexistants sur la plage de Sundays River a été étudiée; il s'agit des Eurydice /ollgicomis (Stüder, 1883), des POllloge/oides laripes (Barnard, 1914 b) et des E.rcirolm/CI llata/cllSis (Vanh6ffen, 1914). -

Ouachita Mountains Ecoregional Assessment December 2003
Ouachita Mountains Ecoregional Assessment December 2003 Ouachita Ecoregional Assessment Team Arkansas Field Office 601 North University Ave. Little Rock, AR 72205 Oklahoma Field Office 2727 East 21st Street Tulsa, OK 74114 Ouachita Mountains Ecoregional Assessment ii 12/2003 Table of Contents Ouachita Mountains Ecoregional Assessment............................................................................................................................i Table of Contents ........................................................................................................................................................................iii EXECUTIVE SUMMARY..............................................................................................................1 INTRODUCTION..........................................................................................................................3 BACKGROUND ...........................................................................................................................4 Ecoregional Boundary Delineation.............................................................................................................................................4 Geology..........................................................................................................................................................................................5 Soils................................................................................................................................................................................................6 -

Occurrence of the Asellid Subfamily Stenasellinae (Crustacea, Isopoda) 1/ in the Western Hemisphere, with Description of a New Genus and Species
Occurrence of the Asellid Subfamily Stenasellinae (Crustacea, Isopoda) 1/ in the Western Hemisphere, with Description of a New Genus and Species-- By Gerald A. Cole and W. L. Minckley Arizona State University, Tempe, Arizona 85281, U. S. A. The taxonomy of the freshwater isopod crustaceans is unsettled, but it seems reasonable at present to divide the widespread fiMily Asellidae into two subfamilies, the Asellinae and the more primitive Stenasellinae. The former includes the North American genus Lirceus Rafinesque 1820, the Old World genus Synasellus Braga 1944, and the abundant Holarctic Asellus G. St.-Hillaire 1764. Subfamily Stenasellinae, proposed by Vandel (1964, translated by Freeman, 1965) and later diagnosed by Magniez (1966b), includes the North African monotypic genus Johannella Monad 1924, and isopods origin- ally assigned to the genus Stenasellus Dollfus 1897. Species of Stenasellus (in the broadest sense) are widely distributed in subterranean waters of Europe, especially in the pen-Mediterranean region (Birstein, 1964; Magniez, 1966b). Twelve forms were recently listed from Europe (Stratraba, 1967), not including S. hazeltoni Collinge (1946) that Chappuis (in Racovitza, 1950) believed to be an oniscoidean isopod, a judgement later confirmed (see Husson, 1957). One species lives in Turkemenia, east-central Asia (Birstein and Starostin, 1949), and at least six species have been described from tropical Africa— Portugese Guinea, the Belgian Congo, and Somalia (Remy, 1938; Monad, 1945; Braga, 1950; Chappuis, 1951, 1952; Lanza, 1966). When Magniez (1966b) diagnosed the subfamily Stenasellinae, he included the Algerian Johanella purpurea Monad and split the old genus Stenasellus into Stenasellus (s. str), Parastenasellus, -2- 1"Studies on which this report are based were supported in part by Grants GB-2461 and GB-6477X from the National Science Foundation, Washington, D. -

SLOVAKIA Caves-Of-Demanova-Valley Slovakia RIS 2006 En
Information Sheet on Ramsar Wetlands (RIS) – 2006-2008 version Available for download from http://www.ramsar.org/ris/key_ris_index.htm. Categories approved by Recommendation 4.7 (1990), as amended by Resolution VIII.13 of the 8 th Conference of the Contracting Parties (2002) and Resolutions IX.1 Annex B, IX.6, IX.21 and IX. 22 of the 9 th Conference of the Contracting Parties (2005). Notes for compilers: 1. The RIS should be completed in accordance with the attached Explanatory Notes and Guidelines for completing the Information Sheet on Ramsar Wetlands. Compilers are strongly advised to read this guidance before filling in the RIS. 2. Further information and guidance in support of Ramsar site designations are provided in the Strategic Framework and guidelines for the future development of the List of Wetlands of International Importance (Ramsar Wise Use Handbook 7, 2 nd edition, as amended by COP9 Resolution IX.1 Annex B). A 3 rd edition of the Handbook, incorporating these amendments, is in preparation and will be available in 2006. 3. Once completed, the RIS (and accompanying map(s)) should be submitted to the Ramsar Secretariat. Compilers should provide an electronic (MS Word) copy of the RIS and, where possible, digital copies of all maps. 1. Name and address of the compiler of this form: Mgr. Dagmar Haviarova, Slovak Caves Administration, FOR OFFICE USE ONLY . DD MM YY Hodzova 11, 031 01 Lipt. Mikulas, Slovakia Tel.: +421/(0)44/5536201, Fax: +421/(0)44/5536311 e-mail: [email protected] RNDr. Zuzana Visnovska, Slovak Caves Administration, Designation date Site Reference Number Hodzova 11, 031 01 Lipt.