Evolution of Carnivory in Lentibulariaceae and the Lamiales
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
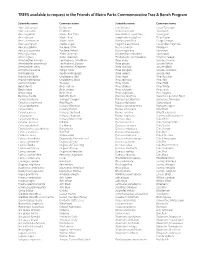
TREES Available to Request in the Friends of Metro Parks Commemorative Tree & Bench Program
TREES available to request in the Friends of Metro Parks Commemorative Tree & Bench Program Scientific name Common name Scientific name Common name Abies balsamaea Fir, Balsam Larix laricina Larch, Tamarack Abies concolor Fir, White Lindera benzoin Spicebush Acer negundo Maple, Box Elder Liquidamber styraciflua Sweetgum Acer rubrum Maple, Red Liriodendron tulipfera Tulip Poplar Acer saccharinum Maple, Silver Maclura pomifera Osage Orange Acer saccharum Maple, Sugar Magnolia acuminata Cucumber magnolia Aesculus glabra Buckeye, Ohio Nyssa sylvatica Blackgum Aesculus octandra Buckeye, Yellow Ostrya viginiana Ironwood Alnus glutinosa Alder, Common Oxydendron arboream Sourwood Alnus rugosa Alder, Speckled Parthenosisis quinquefolia Virginia Creeper Amelanchier arborea Serviceberry, Shadblow Picea abies Spruce, Norway Amelanchier canadensis Serviceberry, Downy Picea glauca Spruce, White Amelanchier laevis Serviceberry Allegheny Picea mariana Spruce, Black Amopha frutocosa Indigo, False Picea pungens Spruce, Colorado Aralia spinosa Devils-walkingstick Picea rubens Spruce, Red Aronia arbutifolia Chokeberry, Red Pinus nigra Pine, Austrian Aronia melancarpa Chokeberry, Black Pinus resinosa Pine, Red Asimina triloba Pawpaw Pinus rigida Pine, Pitch Betula lenta Birch, Yellow Pinus strobus Pine, White Betula lutea Birch, Sweet Pinus sylvestris Pine, Scots Betula nigra Birch, River Pinus virginiana Pine, Virginia Buddeia davidii Butterfly Bush Platanus acerifolia Sycamore, London Plane Campsis radicans Trumpet Creeper Platanus occidentalis Sycamore, -

Outline of Angiosperm Phylogeny
Outline of angiosperm phylogeny: orders, families, and representative genera with emphasis on Oregon native plants Priscilla Spears December 2013 The following listing gives an introduction to the phylogenetic classification of the flowering plants that has emerged in recent decades, and which is based on nucleic acid sequences as well as morphological and developmental data. This listing emphasizes temperate families of the Northern Hemisphere and is meant as an overview with examples of Oregon native plants. It includes many exotic genera that are grown in Oregon as ornamentals plus other plants of interest worldwide. The genera that are Oregon natives are printed in a blue font. Genera that are exotics are shown in black, however genera in blue may also contain non-native species. Names separated by a slash are alternatives or else the nomenclature is in flux. When several genera have the same common name, the names are separated by commas. The order of the family names is from the linear listing of families in the APG III report. For further information, see the references on the last page. Basal Angiosperms (ANITA grade) Amborellales Amborellaceae, sole family, the earliest branch of flowering plants, a shrub native to New Caledonia – Amborella Nymphaeales Hydatellaceae – aquatics from Australasia, previously classified as a grass Cabombaceae (water shield – Brasenia, fanwort – Cabomba) Nymphaeaceae (water lilies – Nymphaea; pond lilies – Nuphar) Austrobaileyales Schisandraceae (wild sarsaparilla, star vine – Schisandra; Japanese -

Trumpet Creeper (Campsis Radicans) Control Herbicide Options
Publication 20-86C October 2020 Trumpet Creeper (Campsis radicans) Control Herbicide Options Dr. E. David Dickens, Forest Productivity Professor; Dr. David Clabo, Forest Productivity Professor; and David J. Moorhead, Emeritus Silviculture Professor; UGA Warnell School of Forestry and Natural Resources BRIEF Trumpet creeper (Campsis radicans), also known as cow itch vine, trumpet vine, or hummingbird vine, is in the Bignoniaceae family and is native to the eastern United States. Trumpet creeper is frequently found in a variety of southeastern United States forests and can be a competitor in pine stands. If not controlled, it can kill the trees it grows on by canopying over the crowns and not allowing adequate sunlight to get to the tree’s foliage for photo- synthesis. Trumpet creeper is a deciduous, woody vine that can “climb” trees up to 40 feet or greater heights (Photo 1) or form mats on shrubs or grows in clumps lower to the ground (Photo 2). The 1 to 4 inch long green leaves are pinnate, ovate in shape and opposite (Photo 3). The orange to red showy flowers are terminal cymes of 4 to 10 found on the plants during late spring into summer (Photo 4). Large (3 to 6 inches long) seed pods are formed on mature plants in the fall that hold hundreds of seeds (Photo 5). Trumpet creeper control is best performed during active growth periods from mid-June to early October in Georgia. If trumpet creeper has climbed up into a num- ber of trees, a prescribed burn or cutting the vines to groundline may be needed to get the climbing vine down to groundline where foliar active herbicides will be effective. -

Evaluating the Adaptive Evolutionary Convergence of Carnivorous Plant Taxa Through Functional Genomics
Evaluating the adaptive evolutionary convergence of carnivorous plant taxa through functional genomics Gregory L. Wheeler and Bryan C. Carstens Department of Evolution, Ecology, & Organismal Biology, The Ohio State University, Columbus, OH, United States of America ABSTRACT Carnivorous plants are striking examples of evolutionary convergence, displaying complex and often highly similar adaptations despite lack of shared ancestry. Using available carnivorous plant genomes along with non-carnivorous reference taxa, this study examines the convergence of functional overrepresentation of genes previously implicated in plant carnivory. Gene Ontology (GO) coding was used to quantitatively score functional representation in these taxa, in terms of proportion of carnivory- associated functions relative to all functional sequence. Statistical analysis revealed that, in carnivorous plants as a group, only two of the 24 functions tested showed a signal of substantial overrepresentation. However, when the four carnivorous taxa were analyzed individually, 11 functions were found to be significant in at least one taxon. Though carnivorous plants collectively may show overrepresentation in functions from the predicted set, the specific functions that are overrepresented vary substantially from taxon to taxon. While it is possible that some functions serve a similar practical purpose such that one taxon does not need to utilize both to achieve the same result, it appears that there are multiple approaches for the evolution of carnivorous function in plant genomes. Our approach could be applied to tests of functional convergence in other systems provided on the availability of genomes and annotation data for a group. Submitted 27 October 2017 Accepted 13 January 2018 Subjects Bioinformatics, Evolutionary Studies, Genomics, Plant Science Published 31 January 2018 Keywords Carnivorous plants, Gene Ontology, Functional genomics, Convergent evolution Corresponding author Gregory L. -

The Fairchild Tropical Garden NIXON SMILEY ______1
~GAZ.NE AMERICAN HORTI CULTURAL SOCIETY A vnion of the Ame'rican Horticultuml Society and the American Ho·rticultural Council 1600 BLADENSB URG ROAD, NORTHEAST . WASHINGTON 2, D. C. For Un ited H mticulture *** to accumulate, increase, and disseminate horticultuml infmmation B. Y. MORRISON, Editor Di?-ec to?'S T enns Expiring 1960 J AMES R. H ARLOW, Managing Editor D ONOVAN S. CORRELL T exas CARL "V. F ENN I NGER Editorial Committee Pennsylvania W. H . HODGE W'. H . HODGE, Chainnan Pen nS)1 Ivan i(~ ] OHN L. CREECH A. J. IRVI NG Yo?'k FREDElRI C P. L EE New "VILLIAM C. STEERE CONRAD B. LI NK New York CURTIS MAY FREDERICK G. MEYER T erms Ex1Jil'ing 1961 STUART M. ARMSTRONG 'WILBUR H. YOUNGMAN Maryland J OHN L. CREECH Maryland Officers 'WILLIAM H . FREDERICK, JR. DelawQ.j·e PR ES IDENT FRANCIS PATTESON-KNIGHT RICHARD P . 'WHITE V il'ginia Washington, D. C. DONALD WYMAN 111 assachv.setts FIRST VICE·PRESIDENT Tenns Expiring 1962 DONALD W YMAN Jamaica Plain, Massachusetts FREDERIC P. LEE Maryland HENRY T. SKINNER SECOND VICE- PRESIDENT Distl'ict of Columba STUART M. ARMSTRONG CEORGE H. SPALDING Silvel' Spring, Mal'yland California RICHARD P. WHITE SECRETARY-TREASURER District of Columbia OLIVE E. WEATHERELL AN NE " VERTSNER WOOD Washington, D. C. Pennsylvania The Amel'ican Ho'yticvltw'al Magazine is the official publication of the American Horticultural Society and is issued fo ur times a year during the q uarters commencing with January, April , July and October. It is devoted to the dissemination of knowledge in the science and art of growing ornamental plants, fruits, vegetables, and related subjects. -

Deletion of Cephalotus Follicularis from Appendix II
Prop. 11.6 CONVENTION ON INTERNATIONAL TRADE IN ENDANGERED SPECIES OF WILD FAUNA AND FLORA A. PROPOSAL Deletion of Cephalotus follicularis from Appendix II. B. PROPONENT Commonwealth of Australia (Environment Australia) C. SUPPORTING STATEMENT The monotypic genus Cephalotus is an insectivorous plant endemic to south western Australia. It occurs on wetland margins throughout the southwest corner of Western Australia. This portion of Western Australia has a high rainfall and as a result there are extensive areas of suitable habitat, especially on the south coastal plain. Within its range are large areas of government owned forests, National Parks and other reserves where the species is common and is likely to occur in vast numbers. The species is easily propagated from small segments of rhizomes and, as a result, it is commonly traded and is widely cultivated. Morphological variation in wild populations is not evident. As the species is easily propagated, it is unlikely that cultivated stocks are derived from wild collections. Cephalotus follicularis has been identified by the CITES Plants Committee under the Ten Year Review as a candidate for deletion from Appendix II as there has been no recorded trade in wild taken specimens since the species was listed. The proposal received full endorsement of the 5th meeting of the CITES Plants Committee in Mexico, May 1994. 1. Taxonomy 1.1. Class Magnoliatae 1.2. Order Rosales 1.3. Family Cephalotaceae 1.4. Genus/species Cephalotus follicularis Labillardière 1806 1.5. Common name Western Australian pitcher plant; Albany pitcher plant 2. Biological data 2.1. Distribution The area of distribution ranges over 400 km from NW to SE and corresponds with the meso- mediterranean climate of the extreme south western part of Australia. -

Wood Anatomy of Buddlejaceae Sherwin Carlquist Santa Barbara Botanic Garden
Aliso: A Journal of Systematic and Evolutionary Botany Volume 15 | Issue 1 Article 5 1996 Wood Anatomy of Buddlejaceae Sherwin Carlquist Santa Barbara Botanic Garden Follow this and additional works at: http://scholarship.claremont.edu/aliso Part of the Botany Commons Recommended Citation Carlquist, Sherwin (1996) "Wood Anatomy of Buddlejaceae," Aliso: A Journal of Systematic and Evolutionary Botany: Vol. 15: Iss. 1, Article 5. Available at: http://scholarship.claremont.edu/aliso/vol15/iss1/5 Aliso, 15(1), pp. 41-56 © 1997, by The Rancho Santa Ana Botanic Garden, Claremont, CA 91711-3157 WOOD ANATOMY OF BUDDLEJACEAE SHERWIN CARLQUIST' Santa Barbara Botanic Garden 1212 Mission Canyon Road Santa Barbara, California 93110-2323 ABSTRACT Quantitative and qualitative data are presented for 23 species of Buddleja and one species each of Emorya, Nuxia, and Peltanthera. Although crystal distribution is likely a systematic feature of some species of Buddleja, other wood features relate closely to ecology. Features correlated with xeromorphy in Buddleja include strongly marked growth rings (terminating with vascular tracheids), narrower mean vessel diameter, shorter vessel elements, greater vessel density, and helical thickenings in vessels. Old World species of Buddleja cannot be differentiated from New World species on the basis of wood features. Emorya wood is like that of xeromorphic species of Buddleja. Lateral wall vessel pits of Nuxia are small (2.5 ILm) compared to those of Buddleja (mostly 5-7 ILm) . Peltanthera wood features can also be found in Buddleja or Nuxia; Dickison's transfer of Sanango from Buddlejaceae to Ges neriaceae is justified. All wood features of Buddlejaceae can be found in families of subclass Asteridae such as Acanthaceae, Asteraceae, Lamiaceae, Myoporaceae, Scrophulariaceae, and Verbenaceae. -

The Miniature Genome of a Carnivorous Plant Genlisea Aurea
Leushkin et al. BMC Genomics 2013, 14:476 http://www.biomedcentral.com/1471-2164/14/476 RESEARCH ARTICLE Open Access The miniature genome of a carnivorous plant Genlisea aurea contains a low number of genes and short non-coding sequences Evgeny V Leushkin1,2, Roman A Sutormin1, Elena R Nabieva1, Aleksey A Penin1,2,3, Alexey S Kondrashov1,4 and Maria D Logacheva1,5* Abstract Background: Genlisea aurea (Lentibulariaceae) is a carnivorous plant with unusually small genome size - 63.6 Mb – one of the smallest known among higher plants. Data on the genome sizes and the phylogeny of Genlisea suggest that this is a derived state within the genus. Thus, G. aurea is an excellent model organism for studying evolutionary mechanisms of genome contraction. Results: Here we report sequencing and de novo draft assembly of G. aurea genome. The assembly consists of 10,687 contigs of the total length of 43.4 Mb and includes 17,755 complete and partial protein-coding genes. Its comparison with the genome of Mimulus guttatus, another representative of higher core Lamiales clade, reveals striking differences in gene content and length of non-coding regions. Conclusions: Genome contraction was a complex process, which involved gene loss and reduction of lengths of introns and intergenic regions, but not intron loss. The gene loss is more frequent for the genes that belong to multigenic families indicating that genetic redundancy is an important prerequisite for genome size reduction. Keywords: Genome reduction, Carnivorous plant, Intron, Intergenic region Background evolutionary and functional points of view. In a model In spite of the similarity of basic cellular processes in eu- plant species, Arabidopsis thaliana, number of protein- karyotes, their genome sizes are extraordinarily variable. -

Carnivorous Plant Responses to Resource Availability
Carnivorous plant responses to resource availability: environmental interactions, morphology and biochemistry Christopher R. Hatcher A doctoral thesis submitted in partial fulfilment of requirements for the award of Doctor of Philosophy of Loughborough University November 2019 © by Christopher R. Hatcher (2019) Abstract Understanding how organisms respond to resources available in the environment is a fundamental goal of ecology. Resource availability controls ecological processes at all levels of organisation, from molecular characteristics of individuals to community and biosphere. Climate change and other anthropogenically driven factors are altering environmental resource availability, and likely affects ecology at all levels of organisation. It is critical, therefore, to understand the ecological impact of environmental variation at a range of spatial and temporal scales. Consequently, I bring physiological, ecological, biochemical and evolutionary research together to determine how plants respond to resource availability. In this thesis I have measured the effects of resource availability on phenotypic plasticity, intraspecific trait variation and metabolic responses of carnivorous sundew plants. Carnivorous plants are interesting model systems for a range of evolutionary and ecological questions because of their specific adaptations to attaining nutrients. They can, therefore, provide interesting perspectives on existing questions, in this case trait-environment interactions, plant strategies and plant responses to predicted future environmental scenarios. In a manipulative experiment, I measured the phenotypic plasticity of naturally shaded Drosera rotundifolia in response to disturbance mediated changes in light availability over successive growing seasons. Following selective disturbance, D. rotundifolia became more carnivorous by increasing the number of trichomes and trichome density. These plants derived more N from prey and flowered earlier. -

Giant Cephalotus of Unknown Origins
Giant Cephalotus of unknown origins Dick Chan • P.O. Box 2252 • Pasadena • California 91102 • USA • [email protected] Introduction I have been growing Cephalotus follicularis for over 20 years. Initially, I was obsessed with grow- ing specimen-type Cephalotus of different clones and to prove once-and-for-all that this was not a difficult plant to grow. Countless plants have met their demise as I experimented with various meth- ods of cultivation. For those that have survived and flourished, I noticed one plant in particular that grew larger, more vigorous, and had a different pitcher/leaf morphology than Cephalotus ‘Hummer’s Giant’ and the typical Cephalotus. However, I do not believe this plant to be just a better-grown speci- men of ‘Hummer’s Giant’. Through the years, I have given and sold this plant to individuals calling it the “Bubble Giant”, however, I have not received nor heard any feedback as to the well-being of those plants. So, for those reading this article and have received this plant from me, I would appreciate seeing some photos. For the remainder of this article, this plant will be referred to as the “unknown”. Origins During my initial spark-of-entry into the hobby, I started collecting Cephalotus cuttings, plants, stems, and leaves from anyone who had the plant and was willing to give or sell a piece to me. Be- cause of that activity, this plant is of an unknown origin because of the feverish pace by which I went about amassing what I had hoped would become a genetically diverse collection of plants. -

A New Carnivorous Plant Lineage (Triantha) with a Unique Sticky-Inflorescence Trap
A new carnivorous plant lineage (Triantha) with a unique sticky-inflorescence trap Qianshi Lina,b,1, Cécile Anéc,d, Thomas J. Givnishc, and Sean W. Grahama,b aDepartment of Botany, University of British Columbia, Vancouver, BC V6T 1Z4, Canada; bUBC Botanical Garden, University of British Columbia, Vancouver, BC V6T 1Z4, Canada; cDepartment of Botany, University of Wisconsin–Madison, Madison, WI 53706; and dDepartment of Statistics, University of Wisconsin–Madison, Madison WI 53706 Edited by Elizabeth A. Kellogg, Donald Danforth Plant Science Center, St. Louis, MO, and approved June 5, 2021 (received for review October 30, 2020) Carnivorous plants consume animals for mineral nutrients that and in wetlands, including bogs, marly shorelines, and calcareous enhance growth and reproduction in nutrient-poor environments. spring-fed fens. In bogs, T. occidentalis is commonly found with Here, we report that Triantha occidentalis (Tofieldiaceae) represents recognized carnivorous plants such as Drosera rotundifolia a previously overlooked carnivorous lineage that captures insects on (Droseraceae) and Pinguicula vulgaris (Lentibulariaceae). During sticky inflorescences. Field experiments, isotopic data, and mixing the summer flowering season, T. occidentalis produces leafless models demonstrate significant N transfer from prey to Triantha, erect flowering stems up to 80 cm tall (12). These scapes have with an estimated 64% of leaf N obtained from prey capture in sticky glandular hairs, especially on their upper portions, a feature previous years, comparable to levels inferred for the cooccurring distinguishing Triantha from other genera of Tofieldiaceae round-leaved sundew, a recognized carnivore. N obtained via carnivory (Fig. 1). Small insects are often found trapped by these hairs; is exported from the inflorescence and developing fruits and may herbarium specimens are frequently covered in insects (Fig. -

Towards Resolving Lamiales Relationships
Schäferhoff et al. BMC Evolutionary Biology 2010, 10:352 http://www.biomedcentral.com/1471-2148/10/352 RESEARCH ARTICLE Open Access Towards resolving Lamiales relationships: insights from rapidly evolving chloroplast sequences Bastian Schäferhoff1*, Andreas Fleischmann2, Eberhard Fischer3, Dirk C Albach4, Thomas Borsch5, Günther Heubl2, Kai F Müller1 Abstract Background: In the large angiosperm order Lamiales, a diverse array of highly specialized life strategies such as carnivory, parasitism, epiphytism, and desiccation tolerance occur, and some lineages possess drastically accelerated DNA substitutional rates or miniaturized genomes. However, understanding the evolution of these phenomena in the order, and clarifying borders of and relationships among lamialean families, has been hindered by largely unresolved trees in the past. Results: Our analysis of the rapidly evolving trnK/matK, trnL-F and rps16 chloroplast regions enabled us to infer more precise phylogenetic hypotheses for the Lamiales. Relationships among the nine first-branching families in the Lamiales tree are now resolved with very strong support. Subsequent to Plocospermataceae, a clade consisting of Carlemanniaceae plus Oleaceae branches, followed by Tetrachondraceae and a newly inferred clade composed of Gesneriaceae plus Calceolariaceae, which is also supported by morphological characters. Plantaginaceae (incl. Gratioleae) and Scrophulariaceae are well separated in the backbone grade; Lamiaceae and Verbenaceae appear in distant clades, while the recently described Linderniaceae are confirmed to be monophyletic and in an isolated position. Conclusions: Confidence about deep nodes of the Lamiales tree is an important step towards understanding the evolutionary diversification of a major clade of flowering plants. The degree of resolution obtained here now provides a first opportunity to discuss the evolution of morphological and biochemical traits in Lamiales.