Chapter 13 Acid-Base Balance
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Pathophysiology of Acid Base Balance: the Theory Practice Relationship
Intensive and Critical Care Nursing (2008) 24, 28—40 ORIGINAL ARTICLE Pathophysiology of acid base balance: The theory practice relationship Sharon L. Edwards ∗ Buckinghamshire Chilterns University College, Chalfont Campus, Newland Park, Gorelands Lane, Chalfont St. Giles, Buckinghamshire HP8 4AD, United Kingdom Accepted 13 May 2007 KEYWORDS Summary There are many disorders/diseases that lead to changes in acid base Acid base balance; balance. These conditions are not rare or uncommon in clinical practice, but every- Arterial blood gases; day occurrences on the ward or in critical care. Conditions such as asthma, chronic Acidosis; obstructive pulmonary disease (bronchitis or emphasaemia), diabetic ketoacidosis, Alkalosis renal disease or failure, any type of shock (sepsis, anaphylaxsis, neurogenic, cardio- genic, hypovolaemia), stress or anxiety which can lead to hyperventilation, and some drugs (sedatives, opoids) leading to reduced ventilation. In addition, some symptoms of disease can cause vomiting and diarrhoea, which effects acid base balance. It is imperative that critical care nurses are aware of changes that occur in relation to altered physiology, leading to an understanding of the changes in patients’ condition that are observed, and why the administration of some immediate therapies such as oxygen is imperative. © 2007 Elsevier Ltd. All rights reserved. Introduction the essential concepts of acid base physiology is necessary so that quick and correct diagnosis can The implications for practice with regards to be determined and appropriate treatment imple- acid base physiology are separated into respi- mented. ratory acidosis and alkalosis, metabolic acidosis The homeostatic imbalances of acid base are and alkalosis, observed in patients with differing examined as the body attempts to maintain pH bal- aetiologies. -

TITLE: Acid-Base Disorders PRESENTER: Brenda Suh-Lailam
TITLE: Acid-Base Disorders PRESENTER: Brenda Suh-Lailam Slide 1: Hello, my name is Brenda Suh-Lailam. I am an Assistant Director of Clinical Chemistry and Mass Spectrometry at Ann & Robert H. Lurie Children’s Hospital of Chicago, and an Assistant Professor of Pathology at Northwestern Feinberg School of Medicine. Welcome to this Pearl of Laboratory Medicine on “Acid-Base Disorders.” Slide 2: During metabolism, the body produces hydrogen ions which affect metabolic processes if concentration is not regulated. To maintain pH within physiologic limits, there are several buffer systems that help regulate hydrogen ion concentration. For example, bicarbonate, plasma proteins, and hemoglobin buffer systems. The bicarbonate buffer system is the major buffer system in the blood. Slide 3: In the bicarbonate buffer system, bicarbonate, which is the metabolic component, is controlled by the kidneys. Carbon dioxide is the respiratory component and is controlled by the lungs. Changes in the respiratory and metabolic components, as depicted here, can lead to a decrease in pH termed acidosis, or an increase in pH termed alkalosis. Slide 4: Because the bicarbonate buffer system is the major buffer system of blood, estimation of pH using the Henderson-Hasselbalch equation is usually performed, expressed as a ratio of bicarbonate and carbon dioxide. Where pKa is the pH at which the concentration of protonated and unprotonated species are equal, and 0.0307 is the solubility coefficient of carbon dioxide. Four variables are present in this equation; knowing three variables allows for calculation of the fourth. Since pKa is a constant, and pH and carbon dioxide are measured during blood gas analysis, bicarbonate can, therefore, be determined using this equation. -

The Renal Response in Man to Acute Experimental Respiratory Alkalosis and Acidosis
The Renal Response in Man to Acute Experimental Respiratory Alkalosis and Acidosis E. S. Barker, … , J. R. Elkinton, J. K. Clark J Clin Invest. 1957;36(4):515-529. https://doi.org/10.1172/JCI103449. Research Article Find the latest version: https://jci.me/103449/pdf THE RENAL RESPONSE IN MAN TO ACUTE EXPERIMENTAL RESPIRATORY ALKALOSIS AND ACIDOSIS 1 BY E. S. BARKER,2, 8 R. B. SINGER,4 J. R. ELKINTON,2 AND J. K. CLARK (From the Renal Section and Chemical Section of the Department of Medicine, The Department of Research Medicine, and the Department of Biochemistry, the University of Pennsylvania School of Medicine, Philadelphia, Pa.) (Submitted for publication August 7, 1956; accepted December 6, 1956) The experimental results to be presented here proximately 30 minutes in 5 of the 6 experiments, and deal with the renal component of the multiple ef- for twice that period in the last experiment; 7.5 to 7.7 fects in man of acute experimental respiratory al- per cent CO, in air or oxygen was inhaled for 21 to 30 minutes. Measurements were continued in both types kalosis (hyperventilation) and acidosis (CO2 in- of experiments during subsequent recovery periods which halation). One aim of the experiments has been ended 97 to 145 minutes after onset of the stimulus (desig- to define an integrated picture of the total body nated time zero). Standard water loading was carried response to acute respiratory acid-base disturb- out before the experiments and continued throughout ances. A previous paper (1) contained a de- with water given in amounts equivalent to urine ex- creted. -

The Electro-Physiology-Feeedback Measures of Interstitial Fluids
INTERNATIONAL MEDICAL UNIVERSITY The elecTro-Physiology-Feeedback Measures oF inTersTiTial Fluids BY PROFESSOR OF MEDICINE DESIRÉ DUBOUNET IMUNE PRESS 2008 Electro-Physiology -FeedBack Measures of Interstitial Fluids edited by Professor Emeritus Desire’ Dubounet, IMUNE ISBN 978-615-5169-03-8 1 CHAPTER 1 THE ELECTRO-PHYSIOLOGY-FEEDBACK MEASURES OF INTERSTITIAL FLUIDS The interstitial liquid constitutes the true interior volume that bathe the organs of the human body. It is by its presence that all the exchanges between plasma and the cells are performed. With the vascular, lymphatic and nervous systems, it seems to be the fourth communication way of information's between all the cells. No direct methods for sampling interstitial fluid are currently available. The composition of interstitial fluid, which constitutes the environment of the cells and is regulated by the electrical process of electrochemistry. This has previously been sampled by the suction blister or liquid paraffin techniques or by implantation of a perforated capsule or wick. The results have varied, depending on the sampling technique and animal species investigated. In one study, the ion distribution between vascular and interstitial compartments agreed with the Donnan equilibrium; in others, the concentrations of sodium and potassium were higher in interstitial fluid than in plasma. The concentration of protein in interstitial fluid is lower than in plasma, and the free ion activities theoretically differ from those of plasma because of the Donnan effect. In spite of these differences, and for practical reasons only, plasma is used clinically to monitor fluid and electrolytes. The relation between plasma and interstitial fluid is important in treating patients with abnormal plasma volume or homeostasis. -

Respiratory Considerations in the Patient with Renal Failure
Respiratory Considerations in the Patient With Renal Failure David J Pierson MD FAARC Introduction Physiologic Connections Between the Lungs and the Kidneys Diseases That Affect Both Lungs and Kidneys Wegener’s Granulomatosis Systemic Lupus Erythematosus Goodpasture’s Syndrome Respiratory Effects of Chronic Renal Failure Pulmonary Edema Fibrinous Pleuritis Pericardial Effusion Tuberculosis and Other Infections Pulmonary Calcification Urinothorax Sleep Apnea Anemia Respiratory Effects of Acute Renal Failure Hemodialysis-Related Hypoxemia How Critical Illness and Mechanical Ventilation Can Damage the Kidneys Summary Lung and kidney function are intimately related in both health and disease. Respiratory changes help to mitigate the systemic effects of renal acid-base disturbances, and the reverse is also true, although renal compensation occurs more slowly than its respiratory counterpart. A large number of diseases affect both the lungs and the kidneys, presenting most often with alveolar hemorrhage and glomerulonephritis. Most of these conditions are uncommon or rare, although three of them— Wegener’s granulomatosis, systemic lupus erythematosus, and Goodpasture’s syndrome—are not infrequently encountered by respiratory care clinicians. Respiratory complications of chronic renal failure include pulmonary edema, fibrinous pleuritis, pulmonary calcification, and a predisposition to tuberculosis. Urinothorax is a rare entity associated with obstructive uropathy. Sleep distur- bances are extremely common in patients with end-stage renal disease, with sleep apnea occurring in 60% or more of such patients. The management of patients with acute renal failure is frequently complicated by pulmonary edema and the effects of both fluid overload and metabolic acidosis. These processes affect the management of mechanical ventilation in such patients and may interfere with weaning. -

Special Propedeutics of Internal Diseases
VITEBSK STATE MEDICAL UNIVERSITY DEPARTMENT OF PROPEDEUTICS OF INTERNAL DISEASES SPECIAL PROPEDEUTICS OF INTERNAL DISEASES LECTURE COURSE Compiled by L.M. Nemtsov, MD (2-е издание) Vitebsk, EI «VSMU» 2016 УДК 616.1/.4-07(07) ББК 54.1 С 71 Рецензенты: директор Белорусского государственного медицинского колледжа доктор медицинских наук И.И. Бураков; профессор кафедры общей и клинической фармакологии Витебского государственного медицинского университета доктор медицинских наук М.Р. Конорев Немцов Л.М. С 71 Special propedeutics of internal diseases : lecture course (Частная пропедевтика внутренних болезней : курс лекций (на английском языке) / Л.М. Немцов. – 2-е изд. – Витебск: ВГМУ, 2016. – 318 с. ISBN 978-985-466-822-2 Курс лекций «Частная пропедевтика внутренних болезней» составлен в соответствии с типовой учебной программой по пропедевтике внутренних болезней, утвержденной Министерством Здравоохранения Республики Беларусь в 1997 г., регистрационный № 08-14/5906, и рабочей учебной программой по пропедевтике внутренних болезней для студентов лечебно-профилактического факультета, утвержденной ВГМУ 29.08.2003 г. по специальности «Лечебное дело». УДК 616.1/.4-07(07) ББК 54.1 Первый выпуск в 2011 г. Немцов Л.М., 2016 УО «Витебский государственный медицинский университет», 2016 ISBN 978-985-466-822-2 CONTENT pp reface 5 Diseases of respiratory system Clinical, laboratory and instrumental methods of diagnostics 6 Basic clinical syndromes of pulmonary diseases 13 Respiratory insufficiency (failure) 18 Bronchitis 21 Pulmonary emphysema 25 Cor pulmonale -

The ABC's of Acid-Base Balance
JPPT REVIEW ARTICLE The ABC’s of Acid-Base Balance Gordon S. Sacks, PharmD The University of Wisconsin—Madison, Madison, Wisconsin A step-wise systematic approach can be used to determine the etiology and proper management of acid-base disorders. The objectives of this article are to: (1) discuss the physiologic processes in- volved in acid-base disturbances, (2) identify primary and secondary acid-base disturbances based upon arterial blood gas and laboratory measurements, (3) utilize the anion gap for diagnostic pur- poses, and (4) outline a stepwise approach for interpretation and treatment of acid-base disorders. Case studies are used to illustrate the application of the discussed systematic approach. KEYWORDS: acid-base J Pediatr Pharmacol Ther 2004;9:235-42 Although acid-base disorders are frequently terms of H+, but due to confusing terminology it encountered in hospital and ambulatory care set- was proposed to convert H+ terminology to pH.1 tings, they are often considered the most difficult When taking the negative logarithm of the H+ areas to understand in medicine. Misdiagnosis due to common misconceptions of acid-base ho- ABBREVIATIONS: AG, Anion gap; HCO3, Bicarbonate; CNS, meostasis often delays identification of the pri- Central nervous system; ECF, Extracellular fluid; Hgb, Hemoglobin; ICU, Intensive care unit; THAM, Tromethamine mary disorder, causing a disruption in the deliv- ery of appropriate therapy. By understanding the concentration, pH represents a measure of H+ basic principles of acid-base physiology, the inter- activity. Optimal function for tissues and organs pretation of acid-base data, and the mechanisms within the human body depends on maintaining responsible for acid-base perturbations, the clini- blood pH between 7.10 and 7.60. -

1960 ANL-6368.Pdf
• • • • • o -" •- - - a - ANL-6368 Biology and Medicine ( TID-4500, 16th Ed., Amended) AEC Research and Development Report ARGONNE NATIONAL LABORATORY 9700 South Cass Avenue Argonne, Illinois BIOLOGICAL AND MEDICAL RESEARCH DIVISION SUMMARY REPORT January through December, 1960 May 1961 Preceding Report: ANL-6264 July through December, 1959 Operated by The University of Chicago under Contract W-31-109-eng-38 with the United States Atomic Energy Commission 1 TABLE OF CONTENTS Page Acute radiation mortality in the parakeet S. P. Stearner, S. A. Tyler, M. H. Sanderson and 5 E. J. Christian ................ ....... • • • • • • • Progress report. Fertility of CF No. 1 female mice irradiated with fission neutrons 10 D. L. Jordan and H. H. Vogel, Jr..... • • • • • • • • 0 • • • • 0 The comparative effectiveness of AET as a protective agent against mortality in neutron- and )'-irradiated mice D. L. Jordan, H. H. Vogel, Jr., N. A. Frigerio, N. Bink, 12 and R. Ba:rhorst ....... ......... .... ... • • • • • • • Life shortening in mice irradiated with either fission neutrons 60 or Co )'-rays at low dose rates H. H. Vogel, Jr., N. A. Frigerio, and D. L. Jordan . 16 Lack of acquired radioresistance after single doses (50 rads) of 60 fission neutrons or Co )'-rays 21 • H. H. Vogel, Jr., and D. L. Jordan ... • • • • • • • • • • • • • • Longevity of female mice irradiated with single sublethal exposures of fission neutrons 27 H. H. Vogel, Jr., and D. L. Jordan.. .......... • • • • • • Mammary tumor incidence in female Sprague-Dawley rats 60 irradiated with either fission neutrons or Co )'-rays. Progress report H. H. Vogel, Jr., and D. L. Jordan. 30 A function for the tissue mast cell 33 L. -
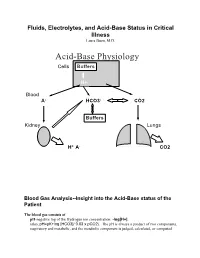
Acid-Base Physiology Cells Buffers
Fluids, Electrolytes, and Acid-Base Status in Critical Illness Laura Ibsen, M.D. Acid-Base Physiology Cells Buffers H+ Blood A- HCO3- CO2 Buffers Kidney Lungs H+ A- CO2 Blood Gas Analysis--Insight into the Acid-Base status of the Patient The blood gas consists of pH-negative log of the Hydrogen ion concentration: -log[H+]. (also, pH=pK+log [HCO3]/ 0.03 x pCO2). The pH is always a product of two components, respiratory and metabolic, and the metabolic component is judged, calculated, or computed by allowing for the effect of the pCO2, ie, any change in the pH unexplained by the pCO2 indicates a metabolic abnormality. - + CO2+H20º H2CO3ºHCO3 + H CO2 and water form carbonic acid or H2CO3, which is in equilibrium with bicarbonate (HCO3-)and hydrogen ions (H+). A change in the concentration of the reactants on either side of the equation affects the subsequent direction of the reaction. For example, an increase in CO2 will result in increased carbonic acid formation (H2CO3) which leads to an increase in both HCO3- and H+ (\pH). Normally, at pH 7.4, a ratio of one part carbonic acid to twenty parts bicarbonate is present in the extracellular fluid [HCO3-/H2CO3]=20. A change in the ratio will affect the pH of the fluid. If both components change (ie, with chronic compensation), the pH may be normal, but the other components will not. pCO2-partial pressure of carbon dioxide. Hypoventilation or hyperventilation (ie, minute ventilation--tidal volume x respitatory rate--imperfectly matched to physiologic demands) will lead to elevation or depression, respectively, in the pCO2. -

Biochemistry for Undergraduates
Prelims.indd 1 27-06-2014 14:46:04 Quick Review of Biochemistry for Undergraduates Questions and Answers Prelims.indd 1 27-06-2014 14:46:04 Prelims.indd 2 27-06-2014 14:46:04 Quick Review of Biochemistry for Undergraduates Questions and Answers Krishnananda Prabhu MD Associate Professor Department of Biochemistry Kasturba Medical College Manipal University Manipal, Karnataka, India Jeevan K Shetty MD Associate Professor Department of Biochemistry RAK College of Medical Sciences Ras Al Khaimah, UAE-SAS The Health Sciences Publishers New Delhi | London | Philadelphia | Panama Prelims.indd 3 27-06-2014 14:46:04 Jaypee Brothers Medical Publishers (P) Ltd Headquarters Jaypee Brothers Medical Publishers (P) Ltd 4838/24, Ansari Road, Daryaganj New Delhi 110 002, India Phone: +91-11-43574357 Fax: +91-11-43574314 Email: [email protected] Overseas Offices J.P. Medical Ltd Jaypee-Highlights Medical Publishers Inc Jaypee Medical Inc 83 Victoria Street, London City of Knowledge, Bld. 237, Clayton The Bourse SW1H 0HW (UK) Panama City, Panama 111 South Independence Mall East Phone: +44-2031708910 Phone: +1 507-301-0496 Suite 835, Philadelphia, PA 19106, USA Fax: +44(0)2030086180 Fax: +1 507-301-0499 Phone: +1 267-519-9789 Email: [email protected] Email: [email protected] Email: [email protected] Jaypee Brothers Medical Publishers (P) Ltd Jaypee Brothers Medical Publishers (P) Ltd 17/1-B Babar Road, Block-B, Shaymali Bhotahity, Kathmandu, Nepal Mohammadpur, Dhaka-1207 Phone: +977-9741283608 Bangladesh Email: [email protected] Mobile: +08801912003485 Email: [email protected] Website: www.jaypeebrothers.com Website: www.jaypeedigital.com © 2014, Jaypee Brothers Medical Publishers The views and opinions expressed in this book are solely those of the original contributor(s)/author(s) and do not necessarily represent those of editor(s) of the book. -

Compensatory Renal Growth After Unilateral Or Subtotal Nephrectomy in the Ovine Fetus
nature publishing group Articles Basic Science Investigation Compensatory renal growth after unilateral or subtotal nephrectomy in the ovine fetus Sébastien Sammut1,2,3, Luc Behr1, Mehrak Hekmati1, Marie Claire Gubler4, Kathleen Laborde5 and Martine Lelièvre Pégorier2,3 BACKGROUND: Clinical and experimental studies show that recorded to be larger than that in normal human. Moreover, unilateral (1/2Nx) and subtotal nephrectomy (5/6Nx) in adults kidney ultrasound studies performed during gestation show result in compensatory renal growth without formation of new that the renal compensation begins before birth (11). In con- nephrons. During nephrogenesis, the response to renal mass trast to the adult, as nephrogenesis is not complete before reduction has not been fully investigated. 36 wk of gestation, enlargement of the remaining kidney could METHODS: Ovine fetuses underwent 1/2Nx, 5/6Nx, or sham involve the formation of new nephrons. In this setting, each surgery (sham) at 70 d of gestation (term: 150 d), when nephro- nephron may have normal dimensions with subnormal single genesis is active. At 134 d, renal function was determined, perfusion and filtration, and the risk of glomeruli injury would fetuses were killed, and kidneys were further analyzed at the be proportionately reduced. Unfortunately, in these studies cellular and molecular levels. Additional fetuses subjected to (9–11), nephron number was not reported. Prevalence of uni- 5/6Nx were killed at 80 and 90 d of gestation to investigate the lateral renal agenesis has been reported in 0.48–0.58 per 1,000 kinetics of the renal compensatory process. live births (12); a 13% decrease in renal function and a 47% RESULTS: At 134 d, in 1/2Nx, a significant increase in kidney increase in hypertension incidence have been reported in adult weight and estimated glomerular number was observed. -

Hyperkalemia
Sodium polystyrene sulfonate is a common treatment serum potassium �6.0 mEq/L were reported. 27 However, for acute hyperkalemia, but there is limited data on larger systematic studies are needed to evaluate the safety its safety and efficacy for the chronic management of and efficacy of sodium polystyrene sulfonate. 1 + hyperkalemia. Long-term use of sorbitol-containing sodium polystyrene sulfonate has been associated with colonic Conclusion necrosis and mucosal injury of the upper gastrointestinal Patients with reduced kidney function due to advanced 24, 25 tract. Researchers also question the efficacy of sodium CKD are at chronic risk for hyperkalemia, and as kidney polystyrene sulfonate in treating hyperkalemia. Sterns et disease progresses and renal function declines, the al found no convincing evidence that sodium polystyrene ability to maintain potassium homeostasis is increasingly sulfonate increases fecal losses of potassium in animal impaired. Hyperkalemia occurs frequently in patients or human studies, and no evidence that adding sorbitol K with CKD treated with RAAS inhibitors, yet these are the to the resin increases its effectiveness in managing same patients who receive the greatest benefit from this hyperkalemia. 26 Conflicting data are reported in a treatment. Currently, therapies indicated for hyperkalemia retrospective chart review of 14 CKD patients on RAAS may have safety and efficacy issues. Therefore, there is a inhibitors treated with daily low-dose sorbitol-free sodium growing need for safe and effective therapies to manage Clinical Update on polystyrene sulfonate after episodes of hyperkalemia. the risk of chronic hyperkalemia. As we learn more about In this small case series, no patients developed colonic this condition in patients with CKD, we are beginning to necrosis or life-threatening events that could be attributed better understand the increasing importance of managing HYPERKALEMIA to sodium polystyrene sulfonate and no recurrences of hyperkalemia over the long term.