MADS-Box Gene Phylogeny and the Evolution of Plant Form
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Updated Checklist of Marine Fishes (Chordata: Craniata) from Portugal and the Proposed Extension of the Portuguese Continental Shelf
European Journal of Taxonomy 73: 1-73 ISSN 2118-9773 http://dx.doi.org/10.5852/ejt.2014.73 www.europeanjournaloftaxonomy.eu 2014 · Carneiro M. et al. This work is licensed under a Creative Commons Attribution 3.0 License. Monograph urn:lsid:zoobank.org:pub:9A5F217D-8E7B-448A-9CAB-2CCC9CC6F857 Updated checklist of marine fishes (Chordata: Craniata) from Portugal and the proposed extension of the Portuguese continental shelf Miguel CARNEIRO1,5, Rogélia MARTINS2,6, Monica LANDI*,3,7 & Filipe O. COSTA4,8 1,2 DIV-RP (Modelling and Management Fishery Resources Division), Instituto Português do Mar e da Atmosfera, Av. Brasilia 1449-006 Lisboa, Portugal. E-mail: [email protected], [email protected] 3,4 CBMA (Centre of Molecular and Environmental Biology), Department of Biology, University of Minho, Campus de Gualtar, 4710-057 Braga, Portugal. E-mail: [email protected], [email protected] * corresponding author: [email protected] 5 urn:lsid:zoobank.org:author:90A98A50-327E-4648-9DCE-75709C7A2472 6 urn:lsid:zoobank.org:author:1EB6DE00-9E91-407C-B7C4-34F31F29FD88 7 urn:lsid:zoobank.org:author:6D3AC760-77F2-4CFA-B5C7-665CB07F4CEB 8 urn:lsid:zoobank.org:author:48E53CF3-71C8-403C-BECD-10B20B3C15B4 Abstract. The study of the Portuguese marine ichthyofauna has a long historical tradition, rooted back in the 18th Century. Here we present an annotated checklist of the marine fishes from Portuguese waters, including the area encompassed by the proposed extension of the Portuguese continental shelf and the Economic Exclusive Zone (EEZ). The list is based on historical literature records and taxon occurrence data obtained from natural history collections, together with new revisions and occurrences. -

Contents of Volume 42 (2012)
CONTENTS OF VOLUME 42 (2012) Issue 1 Tripp-Valdez A., Arreguín-Sánchez F., Zetina-Rejón M.J. The food of Selene peruviana (Actinopterygii: Perciformes: Carangidae) in the southern Gulf of California .................. 1 Zarrad R., Alemany F., Jarboui O., Garcia A., Akrout F. Comparative characterization of the spawning environments of European anchovy, Engraulis encrasicolus , and round sardinella, Sardinella aurita (Actinopterygii: Clupeiformes) in the eastern coast of Tunisia ............................... 9 Ordines F., Valls M., Gouraguine A. Biology, feeding, and habitat preferences of Cadenat’s rockfish, Scorpaena loppei (Actinopterygii: Scorpaeniformes: Scorpaenidae), in the Balearic Islands (western Mediterranean) ..................................... 21 Socha M., Sokołowska-Mikołajczyk M., Szczerbik P., Chyb J., Mikołajczyk T., Epler P. The effect of polychlorinated biphenyls mixture (Aroclor 1254) on the embryonic development and hatching of Prussian carp, Carassius gibelio , and common carp, Cyprinus carpio (Actinopterygii: Cypriniformes: Cyprinidae) ............................................................................................................................................................... 31 Khan A., Ghosh K. Characterization and identification of gut-associated phytase-producing bacteria in some fresh water fish cultured in ponds ...................................................................................................................................................................................... -
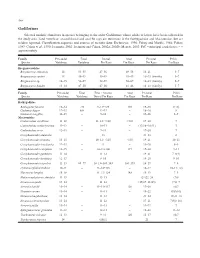
Gadiformes Selected Meristic Characters in Species Belonging to the Order Gadiformes Whose Adults Or Larvae Have Been Collected in the Study Area
548 Gadiformes Selected meristic characters in species belonging to the order Gadiformes whose adults or larvae have been collected in the study area. Total vertebrae, second dorsal and anal fin rays are numerous in the Bathygadidae and Macrouridae, but are seldom reported. Classification sequence and sources of meristic data: Eschmeyer, 1990; Fahay and Markle, 1984; Fahay, 1989; Cohen et al., 1990; Iwamoto, 2002; Iwamoto and Cohen, 2002a; 2002b; Merrett, 2003. PrC = principal caudal rays; ~ = approximately Family Precaudal Total Dorsal Anal Pectoral Pelvic Species Vertebrae Vertebrae Fin Rays Fin Rays Fin Rays Fin Rays Bregmacerotidae Bregmaceros atlanticus 14 53–55 47–56 49–58 16–21 5–7 Bregmaceros cantori 14 45–49 45–49 45–49 16–23 (family) 5–7 Bregmaceros sp. 14–15 52–59 52–59 58–69 16–23 (family) 5–7 Bregmaceros houdei 13–14 47–50 47–50 41–46 16–23 (family) 5–7 Family Precaudal Total First + Second Anal Pectoral Pelvic Species Vertebrae Vertebrae Dorsal Fin Rays Fin Rays Fin Rays Fin Rays Bathygadidae Bathygadus favosus 12–14 ~70 9–11+125 110 15–18 9(10) Gadomus dispar 12–13 80+ 12–13 – 18–20 8 Gadomus longifilis 11–13 – 9–11 – 14–16 8–9 Macrouridae Caelorinchus caribbeus 11–12 – 11–12+>110 >110 17–20 7 Caelorinchus coelorhynchus 11–12 – 10–11 – (17)18–20(21) 7 Caelorinchus occa 12–13 – 9–11 – 17–20 7 Coryphaenoides alateralis – 13 – 21–23 8 Coryphaenoides armatus 13–15 – 10–12+~125 ~135 19–21 10–11 Coryphaenoides brevibarbis 12–13 – 9 – 19–20 8–9 Coryphaenoides carapinus 12–15 – 10–11+100 117 17–20 9–11 Coryphaenoides guentheri -

Genetic Diversity of Antarctic Fish
GENETIC DIVERSITY OF ANTARCTIC FISH Elaine M. Fitzcharles A Thesis Submitted for the Degree of PhD at the University of St Andrews 2014 Full metadata for this item is available in Research@StAndrews:FullText at: http://research-repository.st-andrews.ac.uk/ Please use this identifier to cite or link to this item: http://hdl.handle.net/10023/6860 This item is protected by original copyright Genetic Diversity of Antarctic Fish Elaine M. Fitzcharles This thesis is submitted in partial fulfilment for the degree of PhD at the University of St Andrews May 2014 Supervisor of studies Prof. Alex Rogers (University of Oxford) Dr Melody Clark (British Antarctic Survey) Prof. Andrew Brierley (University of St Andrews) Sponsoring establishment British Antarctic Survey Natural Environment Research Council High Cross Madingley Road Cambridge CB3 0ET United Kingdom ii DECLARATIONS 1. Candidate’s declarations: I, Elaine Fitzcharles, hereby certify that this thesis, which is approximately 35000 words in length, has been written by me, and that it is the record of work carried out by me, or principally by myself in collaboration with others as acknowledged, and that it has not been submitted in any previous application for a higher degree. I was admitted as a research student in September 2005 and as a candidate for the degree of Doctor of Philosophy in April 2007; the higher study for which this is a record was carried out in the University of St Andrews between 2005 and 2014. Date 23/5/11 Signature of candidate …………………….............. 2. Supervisor’s declaration: I hereby certify that the candidate has fulfilled the conditions of the Resolution and Regulations appropriate for the degree of Doctor of Philosophy in the University of St Andrews and that the candidate is qualified to submit this thesis in application for that degree. -

Download Full Article 152.9KB .Pdf File
31 October 1987 Memoirs of the Museum of Victoria 48(1): 75-77 (1987) ISSN 0814-1827 https://doi.org/10.24199/j.mmv.1987.48.17 NEW AUSTRALIAN FISHES. PART 17. NEW SPECIES OF GADELLA AND PHYSICULUS (MORIDAE) By C. D. Paulin Department of Ichthyology, National Museum of New Zealand, Private Bag, Wellington, New Zealand Abstract Paulin, CD., 1987. New Australian fishes. Part 17. New species of Gadella and. Physiculus (Mori- dae). Mem. Mus. Vict. 48: 75-77. The following species are here described: Gadella norops and Physiculus iherosidems. G. norops has a very small light organ placed closer to the anus than the interventral line, elongate ventral fins, second dorsal ray count more than 72 and an inter-orbital width greater than 7.9% SL. P. therosideros has a large light organ placed closer to the anus than the interventral line, equal sized teeth and a lateral line reaching the origin of the second dorsal fin. Introduction Gadella norops sp. nov. Morid fishes of the genera Gadella and Physic- Physiculus edelmanni.-Paulm 1983: 116 (not Brauer, 1908). ulus are distributed in all tropical, subtropical and warm temperate regions of the world's oceans, Material examined. Holotype: Western Australia, Port Hed- in depths of 40-1500 m, usually 100-600 m. Both land (18°41'S, U6°46'E), 506-508 m, 13 Apr 1982, AMS 1. 22827-003. genera are currently being revised on a worldwide Paratypes: New South Wales, off Sydney (33°45'S, basis: Gadella is represented by six nominal and 151°5.5'E), AMS I. -

Palacios-Salgado DS, Burnes-Romo LA, Tavera JJ, Ramírez-Valdez A
3 Palacios-Salgado D.S., Burnes-Romo L.A., Tavera J.J., Ramírez-Valdez A. Endemic fishes of the Cortez Biogeographic Province (eastern Pacific Ocean) .......................................................................... 153 Zogaris S., Chatzinikolaou Y., Koutsikos N., Economou A.N., Oikonomou E., Michaelides G., Hadjisterikotis E., Beaumont W.R.C., Ferreira M.T. Freshwater fish assemblages in Cyprus with emphasis on the effects of dams ............................................................................... 165 Palacios-Salgado D.S., Moreno-Sanchez X.G., Abitia-Cardenas L.A., Gutierrez-Sanchez F.J., Rodriguez-Romero J. Ichthyodiversity of San Jose, San Francisquito, and El Pardito islands in the southwestern Gulf of California, Mexico ......................................................................................................................................................................................................................................... 177 Vasil’eva E.D., Vasil’ev V.P. Fishes of inland waters of the Phu Quoc Island, Gulf of Thailand, Vietnam: ichthyofauna structure and some remarks on the major evolutionary trends in its genesis ................................................................................................................ 193 Fey D.P., Hare J.A. Temperature and somatic growth effects on otolith growth of larval Atlantic menhaden, Brevoortia tyrannus (Actinopterygii: Clupeiformes: Clupeidae) ................................................................................................................... -

Notas Sobre a Biologia De Bathygadus
NOTAS SOBRE A BIOLOGIA DE BATHYGADUS MELANOBRANCHUS VAILLANT, 1888 E GADELLA IMBERBIS (VAILLANT, 1888) (ACTINOPTERYGII, GADIFORMES: BATHYGADIDAE, MORIDAE) AO LARGO DO ESTADO DA BAHIA (NORDESTE DO BRASIL), OCEANO ATLÂNTICO OCIDENTAL Notes about biology of Bathygadus melanobranchus Vaillant, 1888 and Gadella imberbis (Vaillant, 1888) (Actinopterygii, Gadiformes: Bathygadidae, Moridae) off Bahia state (northeastern Brazil), Western Atlantic Ocean JAILZA TAVARES DE OLIVEIRA-SILVA Bióloga. Universidade Estadual de Feira de Santana - Email: [email protected] PAULO ROBERTO DUARTE LOPES Professor assistente. Univ. Est. de Feira de Santana - E-ail: [email protected] 59 GEORGE OLAVO Professor adjunto. Univ. Est. de Feira de Santana - Email:[email protected] Resumo: A alimentação de 15 exemplares de Bathygadus melanobranchus Vail- lant, 1888 (família Bathygadidae, ordem Gadiformes) e de 26 exemplares de Ga- della imberbis (Vaillant, 1888) (família Moridae, ordem Gadiformes) coletados en- tre aproximadamente 11º40´S, 37º13´W e 13º24´S - 38º39´W. B. melanobranchus ingeriu Actinopterygii Teleostei (ocorrência de 100,0%) e Crustacea Decapoda (ocorrência de 50,0%). G. imberbis alimentou-se apenas de Actinopterygii Teleos- tei (ocorrência de 90,0%); escamas de Teleostei (ocorrência de 10,0%) ocorreram apenas em 1 estômago. Palavras-chave: Biologia. Bathygadus melanobranchus. Gadella imberbis Abstract: The feeding of 15 specimens of Bathygadus melanobranchus Vaillant, 1888 (Bathygadidae family, Gadiformes order) and 26 specimens of Gadella imber- bis (Vaillant, 1888) (Moridae family, Gadiformes order) collected between approxi- mately 11º40’S, 37º13’W and 13º24’S - 38º39’W were analyzed. B. melanobranchus ingested Actinopterygii Teleostei (occurrence of 100,0%) and Crustacea Decapoda (occurrence of 50,0%). G. imberbis feed only Actinopterygii Teleostei (occurrence of 90,0%); scales of Teleostei (occurrence of 10,0%) occurred only in 1 stomach. -

An Early Cambrian Fauna of Skeletal Fossils from the Emyaksin Formation, Northern Siberia
An early Cambrian fauna of skeletal fossils from the Emyaksin Formation, northern Siberia ARTEM KOUCHINSKY, STEFAN BENGTSON, SÉBASTIEN CLAUSEN, and MICHAEL J. VENDRASCO Kouchinsky, A., Bengtson, S., Clausen, S. and Vendrasco, M.J. 2015. An early Cambrian fauna of skeletal fossils from the Emyaksin Formation, northern Siberia. Acta Palaeontologica Polonica 60 (2): 421–512. An assemblage of mineralised skeletal fossils containing molluscs, hyoliths, halkieriids, chancelloriids, tommotiids, lo- bopodians, paleoscolecids, bradoriids, echinoderms, anabaritids, hyolithelminths, hexactinnelid, and heteractinid spong- es is described from the early Cambrian Emyaksin Formation exposed along the Malaya Kuonamka and Bol’shaya Kuonamka rivers, eastern flanks of the Anabar Uplift, northern Siberian Platform. The sampled succession is attributed to the Tommotian–Botoman Stages of Siberia and correlated with Stage 2 of Series 1–Stage 4 of Series 2 of the IUGS chronostratigraphical scheme for the Cambrian. Carbon isotope chemostratigraphy is applied herein for regional cor- relation. The fauna contains the earliest Siberian and probably global first appearances of lobopodians, paleoscolecids, and echinoderms, and includes elements in common with coeval faunas from Gondwana, Laurentia, and Baltica. For the first time from Siberia, the latest occurrence of anabaritids is documented herein from the Atdabanian Stage. Problematic calcium phosphatic sclerites of Fengzuella zhejiangensis have not been previously known from outside China. The sel- late sclerites, Camenella garbowskae and mitral sclerites, C. kozlowskii are unified within one species, C. garbowskae. In addition to more common slender sclerites, Rhombocorniculum insolutum include broad calcium phosphatic sclerites. A number of fossils described herein demonstrate excellent preservation of fine details of skeletal microstructures. Based on new microstructural data, sclerites of Rhombocorniculum are interpreted as chaetae of the type occurring in anne- lids. -

New Record of Gadella Jordani and Redescription of Physiculus Japonicus (Pisces: Moridae) in Korea
Anim. Syst. Evol. Divers. Vol. 32, No. 1: 28-37, January 2016 http://dx.doi.org/10.5635/ASED.2016.32.1.028 New Record of Gadella jordani and Redescription of Physiculus japonicus (Pisces: Moridae) in Korea Seo Ha Jang1, Jin-Koo Kim1,*, Jeong-Ho Park2, Young Sun Song1 1Department of Marine Biology, Pukyong National University, Busan 48513, Korea 2National Institute of Fisheries Science, Busan 46083, Korea ABSTRACT We describe the morphological characteristics of two morids, Gadella jordani and Physiculus japonicus, belonging to the order Gadiformes, based on Korean specimens collected from the Korean ocean. Two specimens of Gadella jordani was first collected from Jeju Island, Korea and the East Sea, Korea, in 2013-2014. This species is characterized by 8, 67-69 dorsal fin rays, 66-71 anal fin rays, 5+13 gill rakers, no barbel on the lower jaw, no vomerine teeth, and a ventral luminous organ closer to the anus than to the interventral line. We described it as the first record to the Korean fish fauna, and proposed the new Korean name “Min-su-yeom-dae-gu-sok” for the genus Gadella, and “Min-su-yeom-dae-gu” for the species G. jordani. Physiculus japonicus was first reported by Koh and Moon in the year 1999 based on a single specimen in Korea. However, no study has been attempted to describe the morphological characteristics in Korea since then. In 2013-2014, three specimens of P. japonicus was collected from Jeju Island, Korea and the East Sea, Korea, and we redescribe P. japonicus in detail. This species is characterized by 9-10, 63-64 dorsal fin rays, 70-73 anal fin rays, 3+7-8 gill rakers, a short barbel on the lower jaw, and a ventral luminous organ equidistant between the interventral line and the anus. -

(Actinopterygii: Gadiformes: Moridae) from Southern Japan, with a Note on the Color in Life
Species Diversity, 2010, 15, 131–138 Description of a Pelagic Juvenile Specimen of Gadella jordani (Actinopterygii: Gadiformes: Moridae) from Southern Japan, with a Note on the Color in Life Makoto Okamoto1, Kouji Matsuda2 and Tamaki Matsuda2 1 Seikai National Fisheries Research Institute, 1551-8 Taira-machi, Nagasaki, 851-2213 Japan E-mail: [email protected] 2 Scuba Diving Shop SB, 7641 Shimofukumoto-machi, Kagoshima, 891-0144 Japan (Received 1 July 2010; Accepted 14 September 2010) A pelagic juvenile (43.0 mm standard length) of the morid Gadella jordani (Böhlke and Mead, 1951) was collected from Kagoshima Prefecture, southern Japan. It has a characteristically elongated body, long dorsal and anal fin bases with 73 rays in each fin, the anus located more anteriorly than the origin of the second dorsal fin, a ventral light organ, and no chin barbel. We describe the morphology of this specimen and also present a color photo- graph of it in life. This is the first report of any early life stage in this species. Key Words: Teleostei, Gadiformes, Moridae, Gadella jordani, pelagic juve- nile, color in life, Kagoshima Prefecture, Japan. Introduction Gadella Lowe, 1843 is a genus of morid cod with members distributed from temperate to tropical regions in the deep sea (usually deeper than 150 m) of almost all oceans. They are mainly characterized by having two dorsal fins, a ventral light organ, and no chin barbel (Paulin 1989a, b; Trunov 1992; Paulin and Roberts 1997; Long and McCosker 1998; Sazonov and Shcherbachev 2000). The taxonomy of the genus was reviewed by Paulin (1989b) and Sazonov and Shcherbachev (2000), and 13 species are regarded as valid. -

Order GADIFORMES MACROURIDAE Grenadiers (Rattails) by T
click for previous page Gadiformes: Macrouridae 977 Order GADIFORMES MACROURIDAE Grenadiers (rattails) by T. Iwamoto, California Academy of Sciences, USA iagnostic characters: Small to medium-sized (to about 110 cm in Area 31, commonly between 20 and D60 cm) with laterally compressed body and long, strap-like tail tapering to a slender point. Eye large, 20 to 40% or more of head length; snout in most species prominent, protruding; mouth small to moderately large, jaws subterminal to inferior. Jaw teeth well developed, of variable size and arrangement; no teeth on roof of mouth. Branchiostegal rays 6 or 7. Gill rakers tubercular; outer gill slit greatly restricted by opercular membrane connected to upper and lower reaches of gill arch. Two dorsal fins, the first short-based and high, with second ray spinous; second dorsal fin long-based, confluent with anal fin at end of tail; anal fin usually with much longer rays than second dorsal fin; no caudal fin; pelvic fin usually situated forward of pectoral-fin origin, 7 to 14 rays in species from Area 31.Exposed field of scales in almost all species covered with spinules; many with modified, thick, spiny scales at tip of snout and over ridges of head. Colour: variably brown, black, grey, bluish, often silvery along sides of head and body. 2nd ray spinous, st often serrated on scales 1 ray spinous, leading edge usually with small, closely spinules no caudal fin, 2nd dorsal and anal fins joined urogenital no teeth on roof anal-fin rays opening of mouth usually longer than dorsal-fin light organ in some species ventral view Habitat, biology,and fisheries: Benthopelagic fishes of continental slope and rise, in about 250 to more than 4 000 m (a few species pelagic, but none in the area). -

Fishes of the World
Fishes of the World Fishes of the World Fifth Edition Joseph S. Nelson Terry C. Grande Mark V. H. Wilson Cover image: Mark V. H. Wilson Cover design: Wiley This book is printed on acid-free paper. Copyright © 2016 by John Wiley & Sons, Inc. All rights reserved. Published by John Wiley & Sons, Inc., Hoboken, New Jersey. Published simultaneously in Canada. No part of this publication may be reproduced, stored in a retrieval system, or transmitted in any form or by any means, electronic, mechanical, photocopying, recording, scanning, or otherwise, except as permitted under Section 107 or 108 of the 1976 United States Copyright Act, without either the prior written permission of the Publisher, or authorization through payment of the appropriate per-copy fee to the Copyright Clearance Center, 222 Rosewood Drive, Danvers, MA 01923, (978) 750-8400, fax (978) 646-8600, or on the web at www.copyright.com. Requests to the Publisher for permission should be addressed to the Permissions Department, John Wiley & Sons, Inc., 111 River Street, Hoboken, NJ 07030, (201) 748-6011, fax (201) 748-6008, or online at www.wiley.com/go/permissions. Limit of Liability/Disclaimer of Warranty: While the publisher and author have used their best efforts in preparing this book, they make no representations or warranties with the respect to the accuracy or completeness of the contents of this book and specifically disclaim any implied warranties of merchantability or fitness for a particular purpose. No warranty may be createdor extended by sales representatives or written sales materials. The advice and strategies contained herein may not be suitable for your situation.