Page 1 “OLD-GOLD” FLOWER COLOR, the SECOND CASE OF
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Thermoflex Pantone Swatches
Pantone Color Match NOTE: Color match is approximate and can vary by manufacture batch ALTERNATIVE THERMOFLEX PLUS PART # PANTONE # PANTONE # THERMOFLEX XTRA PART # PANTONE # WHITE PLS-9100 N/A WHITE TFX-8100 N/A ICE GREY PLS-9120 428C STORM GREY TFX-8150 430C STORM GREY PLS-9150 430C BLACK TFX-8236 3C DARK GREY PLS-9152 425C RED TFX-8301 200C BLACK PLS-9236 3C HOT PINK TFE-8310 215C RED PLS-9301 200C ORANGE TFX-8333 165C ORCHID PINK PLS-9304 493C MAROON TFX-8350 229C DUSTY ROSE PLS-9305 210C ATHLETIC GOLD TFX-8426 1235C MEDIUM PINK PLS-9307 189C NAVY BLUE TFX-8513 2767 ROSA PLS-9308 214C ROYAL BLUE TFX-8522 301 CORAL PLS-9309 177C COLUMBIA BLUE TFX-8576 299 HOT PINK PLS-9310 215C ROYAL PURPLE TFX-8584 2755 CRIMSON PLS-9312 216C KELLY GREEN TFX-8633 342C FLAME RED PLS-9315 032C FOREST GREEN TFX-8676 553C ORANGE PLS-9333 165C ANTIQUE SILVER TFX-8834 877C TANGERINE PLS-9335 1585C OLD GOLD TFX-8843 871C PEACH PLS-9337 1565C SALMON PLS-9338 171C TEXAS ORANGE PLS-9340 1605C ECONOMYFLEX PART # PANTONE # MAROON PLS-9350 229C BLACK EF-01 3C VIOLET PLS-9360 249C WHITE EF-02 N/A ATHLETIC GOLD PLS-9426 1235C RED EF-03 200C MEDIUM YELLOW PLS-9450 116C 7502C ATHLETIC GOLD EF-04 1365C VEGAS GOLD PLS-9460 467C 465C NAVY EF-05 533C OCHRE PLS-9465 874C 394C GREY EF-06 422C LEMON YELLOW PLS-9472 3955C SKY BLUE EF-07 7454C BRIGHT LEMON PLS-9473 102C 296C KELLY GREEN EF-08 342C NAVY BLUE PLS-9513 2767 ROYAL BLUE EF-09 287C REFLEX BLUE PLS-9519 2746C ATH. -

Opelousas, La
JACOBS NEWS DEPOT COMPANY, OPELOUSAS, LA. SEPTEMBER MARM Our School Supplies Now Ready SJacobs Special Dictionaries School Teachers Self-Filler 25c. 50c, 75c, and $1.25 Bags, Boxes, Inks Our 50c Values Pound Paper FOUNTAIN PEN Lunch Wax Papers Pencils, Erasers, Pens Correspondenc Cards and $2.00 Paper Napkins Tablet, Scisors Box Paper JACOBS Drinking Cups Will Satisy You Always Sharp Paper Towels Composition Books Palmer Pens PAL PENCIL Palmer Paper $1.00 Toilet Papers Rulers, Crayolas Note Book : terling Silver 3.00 Black Board Examination Tablets Covers, Drawing SWAT MANS 0 Cloth and Erasers Paste, Dust Pans a Skull aps WATERMANS Chalk Skull Cape FOUNTAIN PENS ak Dusters, Pokers The Good Kind .. •,..•. o •-• B . *j 2.50 and Up JACOBS' SPECIAL C ai Shovels Black and Orange SJACOBS' SPECIAL 25c Waste Baskets Green and Gold STwo Blades Indelible and Purple and White S pocket Knives 25c Composition Books Pountain Pen Inks Purple and Old Gold 25c ... m .. ...... Shbt' Special Waterproof Bags for Boys or Girls 35cts Special prices to Merchants Mallet; South, by Arestide Gull I fItONS PICK SEATTLEL IRLS FOR BEAUTY lory; East, by Samuel Guillory, Jr.; PERSONAL and West, by Mrs. Manuel Guillory, and being same property acquiied by Mr. Moresi and Mr. Schexnayder of Leopold Papillon from Cleophas Guil. Jeanerette, the former being a lory as GOPYVUent is* AJo1okSTiA-* per act passed before J. R nephew of Jack Morasi Pavy, Notary Public, on the 28th day of this city, of October, 1918. were here Wednesday, haing motored TERMS AND OOND)PIONS-Cash to Opelousas to witness the ball cille Dunbar and Messrs. -

Golden Holidaydesigned by Mike Vickery
© Copyright 2001 Kreinik Mfg. Co., Inc. All rights reserved. Kreinik Consumer Info: 1-800-537-2166 or www.kreinik.com, email [email protected] GOLDEN HOLIDAY DESIGNED BY MIKE VICKERY DESIGN SIZE: 127WX91H Materials needed: • Kreinik Metallic Gift Collection: Gold • 14-count Aida cloth in cream color • DMC embroidery floss as listed in color key Instructions: Cross stitch this design centered on your fabric. Use two strands of floss or one strand of Kreinik Braid according to color key. Each square on the chart equals one fabric thread. Color Key is below: DMC White DMC 304 Md. Christmas Red DMC 310 Black On the packages in the sled: DMC 402 Very Light Mahogany Straight stitch with 1/16” Ribbon 202HL Aztec Gold DMC 435 Very Light Brown Straight stitch with Gold Ombre (red package) Kreinik Fine (#8) Braid 221 Antique Gold DMC 644 Medium Beige Grey DMC 666 Bright Christmas Red DMC 676 Light Old Gold Kreinik Very Fine (#4) Braid 102 Vatican Gold DMC 700 Bright Christmas Green DMC 702 Kelly Green DMC 704 Bright Chartreuse DMC 796 Dark Royal Blue Kreinik Fine (#8) Braid 210 Gold Dust DMC 3705 Dark Melon DMC 3046 Medium Yellow Beige DMC 822 Light Beige Grey DMC 798 Dark Delft Blue DMC 3776 Light Mahogany Stitch a bow at the dots on the two packages using1/16” Ribbon on the blue box, and a bow with Ombre on the red box Kreinik Japan #7 002J Gold laid on the surface of the fabric and couched with 002C Gold Cord Backstitch with DMC 3799 Very Dark Pewter Grey (1 strand, except for base of sled where we recommend two strands) Backstitch horn with 002C Gold Cord or Very Fine (#4) Braid 002. -
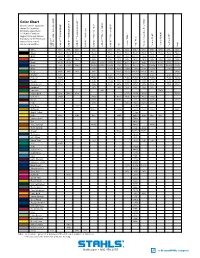
Color Chart ® ® ® ® Closest Pantone® Equivalent Shown
™ ™ II ® Color Chart ® ® ® ® Closest Pantone® equivalent shown. Due to printing limitations, colors shown 5807 Reflective ® ® ™ ® ® and Pantone numbers ® ™ suggested may vary from ac- ECONOPRINT GORILLA GRIP Fashion-REFLECT Reflective Thermo-FILM Thermo-FLOCK Thermo-GRIP ® ® ® ® ® ® ® tual colors. For the truest color ® representation, request Scotchlite our material swatches. ™ CAD-CUT 3M CAD-CUT CAD-CUT CAD-CUT CAD-CUT CAD-CUT CAD-CUT Felt Perma-TWILL Poly-TWILL Thermo-FILM Thermo-FLOCK Thermo-GRIP Vinyl Pressure Sensitive Poly-TWILL Sensitive Pressure CAD-CUT White White White White White White White White White* White White White White White Black Black Black Black Black Black Black Black Black* Black Black Black Black Black Gold 1235C 136C 137C 137C 123U 715C 1375C* 715C 137C 137C 116U Red 200C 200C 703C 186C 186C 201C 201C 201C* 201C 186C 186C 186C 200C Royal 295M 294M 7686C 2747C 7686C 280C 294C 294C* 294C 7686C 2758C 7686C 654C Navy 296C 2965C 7546C 5395M 5255C 5395M 276C 532C 532C* 532C 5395M 5255C 5395M 5395C Cool Gray Warm Gray Gray 7U 7539C 7539C 415U 7538C 7538C* 7538C 7539C 7539C 2C Kelly 3415C 341C 340C 349C 7733C 7733C 7733C* 7733C 349C 3415C Orange 179C 1595U 172C 172C 7597C 7597C 7597C* 7597C 172C 172C 173C Maroon 7645C 7645C 7645C Black 5C 7645C 7645C* 7645C 7645C 7645C 7449C Purple 2766C 7671C 7671C 669C 7680C 7680C* 7680C 7671C 7671C 2758U Dark Green 553C 553C 553C 447C 567C 567C* 567C 553C 553C 553C Cardinal 201C 188C 195C 195C* 195C 201C Emerald 348 7727C Vegas Gold 616C 7502U 872C 4515C 4515C 4515C 7553U Columbia 7682C 7682C 7459U 7462U 7462U* 7462U 7682C Brown Black 4C 4675C 412C 412C Black 4C 412U Pink 203C 5025C 5025C 5025C 203C Mid Blue 2747U 2945U Old Gold 1395C 7511C 7557C 7557C 1395C 126C Bright Yellow P 4-8C Maize 109C 130C 115U 7408C 7406C* 7406C 115U 137C Canyon Gold 7569C Tan 465U Texas Orange 7586C 7586C 7586C Tenn. -

Thread Color Chart Digital
Embroidered Emblem Thread Colour Chart Thread Fils Charte des couleurs de fil pour les emblèmes brodés Dk. Red 508 Cream 004 Maroon 035 Ultraviolet 538 MA 1981 / IS 1912 MA 1723 / IS 0651 V MA 1983 / IS 2944 MA 1634 / IS 3114 PMS® 1955 C PMS® 7506 C PMS® 5185 C PMS® 2695 C Red 032 Maize 536 Wine 034 Dk. Navy 025 MA 1839 / IS 1902 MA 1826 / IS 0811 V MA 1635 / IS 2222 V MA 1844 / IS 3344 V PMS® 187 C PMS® 1355 C PMS® 222 C PMS® 5395 C Foxy Red 201 Amberlite 073 Russet 206 Lt. Navy 024 MA 1838 / IS 1904 MA 1673 / IS 0832 V MA 1784 / IS 2113 MA 1966 / IS 3323 V PMS® 200 C PMS® 465 C PMS® 7638 C PMS® 2766 C Spanish Red 053 Sand 003 Cranberry 217 Imperial Blue 070 MA 1637 / IS 1704 MA 1939 / IS 0643 V MA 1781 / IS 2011 MA 1967 / IS 3323 PMS® 1795 C PMS® 7503 C PMS® 207 C PMS® 281 C Warm Red 082 Marine Gold 039 Flesh Pink 526 Steel Blue 521 MA 1878 / IS 1703 MA 1792 / IS 0542 V MA 1656 / IS 1760 V MA 1764 / IS 3444 PMS® 485 C PMS® 118 C PMS® 480 C PMS® 534 C Neon Orange 503 Old Gold 096 Lt. Pink 030 Royal Blue 023 MA 1837 / IS 1306 V MA 1672 / IS 0822 V MA 1815 / IS 2363 MA 1767 / IS 3333 PMS® 811 C PMS® 126 C PMS® 1895 C PMS® 2746 C Tropical Orange 534 Tiger Eye 535 Pink 523 Sapphire 520 MA 1987 / IS 1305 V MA 1791 / IS 0822 V MA 1921 / IS 2650 MA 1676 / IS 3543 PMS® 1665 C PMS® 125 C PMS® 210 C PMS® 7687 C Dk. -
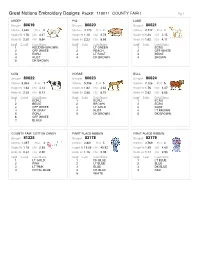
J9 Packline Report V8
Great Notions Embroidery Designs Pack#: 110011 COUNTY FAIR I Pg 1 SHEEP PIG LAMB Design# 80619Design# 80620Design# 80621 Stitches 4,622 #Col 5 Stitches 4,170 #Col 4 Stitches 2,747 #Col 4 Height IN 1.76 CM 4.47 Height IN 1.10 CM 2.79 Height IN 1.24 CM 3.15 Width IN 2.30 CM 5.66 Width IN 2.23 CM 5.66 Width IN 1.62 CM 4.11 Seq# Color# Color Name Seq# Color Color Name Seq# Color Color Name 1 REDDISH BROWN 1 LT GREEN 1 ECRU 2 OFF WHITE 2 PEACH 2 OFF WHITE 3 ECRU 3 LT RUST 3 BEIGE 4 RUST 4 DK BROWN 4 BROWN 5 DK BROWN COW HORSE BULL Design# 80622Design# 80623Design# 80624 Stitches 5,834 #Col 7 Stitches 5,756 #Col 5 Stitches 7,126 #Col 5 Height IN 1.63 CM 4.14 Height IN 1.82 CM 4.62 Height IN 1.76 CM 4.47 Width IN 2.35 CM 6.73 Width IN 2.65 CM 6.73 Width IN 2.62 CM 6.65 Seq# Color# Color Name Seq# Color Color Name Seq# Color Color Name 1 ECRU 1 ECRU 1 ECRU 2 BEIGE 2 BROWN 2 ECRU 3 OFF WHITE 3 LT GOLD 3 RUST 4 DK GRAY 4 RUST 4 LT BROWN 5 ECRU 5 DK BROWN 5 DK BROWN 6 OFF WHITE 7 BLACK 'COUNTY FAIR' COTTON CANDY FIRST PLACE RIBBON FIRST PLACE RIBBON Design# 81325Design# 83178Design# 83179 Stitches 1,397 #Col 4 Stitches 3,801 #Col 5 Stitches 3,769 #Col 4 Height IN 1.16 CM 2.95 Height IN 18.08 CM 45.92 Height IN 1.89 CM 4.80 Width IN 2.34 CM 2.95 Width IN 1.16 CM 2.95 Width IN 1.14 CM 2.90 Seq# Color# Color Name Seq# Color Color Name Seq# Color Color Name 1 LT GOLD 1 DK BLUE 1 LT BLUE 2 PINK 2 LT BLUE 2 BLUE 3 LT PINK 3 BLUE 3 DK BLUE 4 ROYAL BLUE 4 DK BLUE 4 RED 5 WHITE Great Notions Embroidery Designs Pack#: 110011 COUNTY FAIR I Pg 2 FIRST PLACE -

Embroidery Color Chart.Indd
ARTWORK GUIDE SCREEN PRINTING DIRECT-TO-GARMENT EMBROIDERY POSTER PRINTING MUG PRINTING PHONE CASE PRINTING SCREEN PRINTING Screen printing, sometimes referred to as silk screening, is a form of garment printing in which plastisol-based inks are pressed through a screen onto a garment, leaving a layer of ink on top of the fabric. Screen printing is the most established and widely-used form of garment printing available. During the printing process, a set of screens (one for each color being printed) rotates around a press in a carousel motion and takes turns being pressed onto each garment. We require vector EPS files to be submitted for screenprint orders. Artwork guidelines All artwork submissions for screen printing must be EPS files. All graphics must be fully vectorized. All text must be outlined. Use spot colors only - match one of our 32 base colors (listed below) or a Pantone color. Separate artwork files required to print on multiple sides of garment. Print specifications The Scalable Press API requires you specify which inks will be used for screenprint designs. You can specify any coated Pantone color (from a range of 100 - 7774 C) or choose one of our standard ink colors. 32 base colors: Athletic Gold Black Blue Brown Cardinal Charity Dark Grey Forest Grey Hot Pink Kelly Green Lemon Light Blue Light Grey Light Purple Lime Magenta Maroon Navy Old Gold Olive Orange Orange Red Pink Purple Red Royal Tan Turquoise White Green Yellow Pantone matching costs $5 per color. DIRECT-TO-GARMENT (DTG) Direct to garment (or DTG) printing involves an advanced inkjet printer laying ink directly onto a garment. -

How to Identify Old Chinese Porcelain
mmmKimmmmmmKmi^:^ lOW-TO-IDENTIFY OLD -CHINESE - PORCELAIN - j?s> -ii-?.aaig3)g'ggg5y.jgafE>j*iAjeE5egasgsKgy3Si CORNELL UNIVERSITY LIBRARY THE WASON CHINESE COLLECTION DATE DUE 1*-^'" """"^*^ NK 4565!h69" "-ibrary 3 1924 023 569 514 The original of this book is in the Cornell University Library. There are no known copyright restrictions in the United States on the use of the text. http://www.archive.org/details/cu31924023569514 'a4^(A<-^^ %//3 HOW TO IDENTIFY OLD CHINESE PORCELAIN PLATE r WHITE PORCELAIN "BLANC-DE-CHINE" PAIR OF BOWLS of pierced fret-work divided by five circular panels or medallions of raised figures in relief, supposed 10 represent the Pa-Sien or eight Immortals and the God of Longevity. Height, if in. Diameter, sfin. SEAL in the form of a cube surmounted by the figure of a lion Height, i^in. INCENSE BURNER, eight sided and ornamented by moulding in relief with eight feet and four handles. The sides have three bands enclosing scrolls in ancient bronze designs. At each angle of the cover is a knob; it is ornamented with iris and prunus, and by pierced spaces. The stand has eight feet and a knob at each angle ; in the centre is a flower surrounded by detached impressed scrolls, round the outside are similar panels to those on the bowl. Height, 4|in. Diameter of stand, 6f in. THE FIGURE OF A CRAB on a lotus leaf, the stem of which terminales in a flower. Length, 6| in. From Sir PV. fraiik^s Collection at the BritisJi Museum. S3 HOW TO IDENTIFY OLD CHINESE PORCELAIN BY MRS. -

CLASSIC RAYON 100% Viscose
CLASSIC RAYON 100% viscose While every attempt is made to reproduce thread colors accurately, colors on your monitor or print out may not precisely match thread colors. Color names are for your reference only. When ordering please refer to each color by color number. 1013 Peach Blush 1111 Evening Mist 1015 Desert Bloom 1031 Frosted Lavender 1317 Blush Pink 1235 Crocus 1220 Conch Shell 1320 Purple Heart 1307 Raspberry Punch 1388 Plum 1485 Electric Red 1319 Iris 1039 Brick Red 1488 Dark Magenta 1038 Barn Red 1310 Magenta 1114 Pink Petal 1321 Bubble Gum Pink 1115 Powder Puff 1121 Candy Heart 1315 Pink Grapefruit 1309 Dahlia 1148 Rustic Pink 1109 Pink Rose 1384 Merlot 1110 Fuchsia 1385 Garnet 1383 Pink Pansy 1182 Mulberry 1187 Orchid 1281 Radish 1234 Hibiscus 1184 Scarlet Rose 1117 Flamingo Pink 1154 Lipstick Rose 1183 Cranberry 1107 Honeysuckle 1389 Bordeaux 1014 Bermuda Sand 1034 Vintage Rose 1120 Baby Pink 1119 English Rose 1116 Cotton Candy 1035 Burgundy 1108 Pink Carnation 1386 Eggplant 1354 Watermelon 1356 Pink Pearl 1081 Azalea 1141 Mauve 1186 Ruby Slipper 1382 Colonial Rose 1381 Ripe Raspberry 1236 Plum Brandy 3 4 CLASSIC RAYON 100% viscose While every attempt is made to reproduce thread colors accurately, colors on your monitor or print out may not precisely match thread colors. Color names are for your reference only. When ordering please refer to each color by color number. 1261 Lavendula 1198 Moonstone 1266 Regal Blue 1030 Light Periwinkle 1166 Hanukkah Blue 1364 Storm Sky Blue 1466 Sailor Blue 1365 Dusty Plum 1335 Dark Periwinkle -

Air Force Blue (Raf) {\Color{Airforceblueraf}\#5D8aa8
Air Force Blue (Raf) {\color{airforceblueraf}\#5d8aa8} #5d8aa8 Air Force Blue (Usaf) {\color{airforceblueusaf}\#00308f} #00308f Air Superiority Blue {\color{airsuperiorityblue}\#72a0c1} #72a0c1 Alabama Crimson {\color{alabamacrimson}\#a32638} #a32638 Alice Blue {\color{aliceblue}\#f0f8ff} #f0f8ff Alizarin Crimson {\color{alizarincrimson}\#e32636} #e32636 Alloy Orange {\color{alloyorange}\#c46210} #c46210 Almond {\color{almond}\#efdecd} #efdecd Amaranth {\color{amaranth}\#e52b50} #e52b50 Amber {\color{amber}\#ffbf00} #ffbf00 Amber (Sae/Ece) {\color{ambersaeece}\#ff7e00} #ff7e00 American Rose {\color{americanrose}\#ff033e} #ff033e Amethyst {\color{amethyst}\#9966cc} #9966cc Android Green {\color{androidgreen}\#a4c639} #a4c639 Anti-Flash White {\color{antiflashwhite}\#f2f3f4} #f2f3f4 Antique Brass {\color{antiquebrass}\#cd9575} #cd9575 Antique Fuchsia {\color{antiquefuchsia}\#915c83} #915c83 Antique Ruby {\color{antiqueruby}\#841b2d} #841b2d Antique White {\color{antiquewhite}\#faebd7} #faebd7 Ao (English) {\color{aoenglish}\#008000} #008000 Apple Green {\color{applegreen}\#8db600} #8db600 Apricot {\color{apricot}\#fbceb1} #fbceb1 Aqua {\color{aqua}\#00ffff} #00ffff Aquamarine {\color{aquamarine}\#7fffd4} #7fffd4 Army Green {\color{armygreen}\#4b5320} #4b5320 Arsenic {\color{arsenic}\#3b444b} #3b444b Arylide Yellow {\color{arylideyellow}\#e9d66b} #e9d66b Ash Grey {\color{ashgrey}\#b2beb5} #b2beb5 Asparagus {\color{asparagus}\#87a96b} #87a96b Atomic Tangerine {\color{atomictangerine}\#ff9966} #ff9966 Auburn {\color{auburn}\#a52a2a} #a52a2a Aureolin -

Beautiful Old Chinese Rugs
m Digitized by the Internet Archive in 2013 http://archive.org/details/beautifuloldchinOOamer ON FREE PUBLIC VIEW AT THE AMERICAN ART GALLERIES MADISON SQUARE SOUTH, NEW YORK BEGINNING ON NEW YEAR'S DAY, 1915 AND CONTINUING UNTIL THE DATE OF PUBLIC SALE A VERY NOTABLE COLLECTION OF ANTIQUE CHINESE RUGS TO BE SOLD AT UNRESTRICTED PUBLIC SALE AT THE AMERICAN ART GALLERIES ON WEDNESDAY, THURSDAY, FRIDAY AND SATURDAY AFTERNOONS, JANUARY 6th, 7th, 8th AND 9th BEGINNING EACH AFTERNOON AT 2.30 O'CLOCK No. 526—Antique Chinese Salmon Pink Rug ILLUSTRATED CATALOGUE OF THE VERY NOTABLE COLLECTION OF BEAUTIFUL OLD CHINESE RUGS GATHERED DURING MANY YEARS BY THE WIDELY KNOWN CONNOISSEUR MR. THOMAS B. CLARKE TO BE SOLD AT UNRESTRICTED PUBLIC SALE AT THE AMERICAN ART GALLERIES ON THE AFTERNOONS HEREIN STATED CATALOGUE WRITTEN BY MR. DANA H. CARROLL THE SALE WILL BE CONDUCTED BY MR. THOMAS E. KIRBY AND HIS ASSISTANT. MR. OTTO BERNET, OF THE AMERICAN ART ASSOCIATION, Managers 1915 THE AMERICAN ART ASSOCIATION DESIGNS ITS CATALOGUES AND DIRECTS ALL DETAILS OF ILLUSTRATION TEXT AND TYPOGRAPHY A FOREWORD It was a few years ago only that Americans awoke to the beauties of the antique Chinese rug. Rugs of the nearer Orient had then already been acquired here with such appetite that it was credibl>' stated that there were more of them in this country than in any other. But even those persons who posed as accom- plished purveyors of the artistic })roductions of Cathay had failed to feel the potency of charm inherent in these creations of the Celestial looms. -

COLONIAL GEORGIAN 1700-1776 FEDERAL 1780-1820 Base Color Trim Color Door Color Brick White Black Off-White Buff Natural Pale Ye
COLONIAL GEORGIAN 1700-1776 Base Color Trim Color Door Color Natural Same as Base Dark Brown Spanish Brown (dark, dul red) White Black/Green Prussian (Dark Blue/Green) Dark Grey Indian Red ("verging on scarlet") Dark Red Yellow Orche Green FEDERAL 1780-1820 Base Color Trim Color Door Color Brick White Black Off-White Buff Natural Pale Yellow Medium Blue Brown Ochre White Pale Yellow White Red Soft Beige Pale Green Medium Grey Medium Blue GOTHIC REVIVAL 1850-1870 TO EARLY VICTORIAN Base Color Trim Color Door Color Shades of Grey Darker than Base Unpainted Wood Drab or Fawn Darker than Base Oak Sage Lighter than Base Straw/Sand Lighter than Base Chocolate Red Buff Dark Grey Brick Pink Dark Green/Brown Mustard Straw Colored Stucco BRACKETED OR ITALIANATE 1840-1880 Base Color Trim Color Door Color Pale Beige Darker Beige Black Golden Sand Lighter Sand Natural Golden Brown Darker Brown Burgundy Olive Branch Stain Lighter Olive Light Grey Dark Grey Deep Grey Light Grey Grey Stain Lighter Stain Yellow Orche Dark Green Blue Grey Medium Brown Stone Dark Brown Old Gold Medium Red Fawn Sash (Reddish Brown) Buff Shutter Green MANSARD or SECOND EMPIRE 1855-1885 Base Color Trim Color Door Color Pale Olive Ivory Olive Rose Pale Rose Dark Green Peach Pale Peach Golden Sands Stain Ivory/Yellow Sash Olive Tan Bittersweet Straw Cream/Yellow Sash Light Yellow White/Brown Brown Brown/Bittersweet Sash & Shutters Light Brownstone Medium Brownstone QUEEN ANNE 1875-1905 MULTICOLORED PERIOD Base Color Trim Color Door Color Light Olive Dark Olive/Dark Red Accent