Experiment 2: Using Bomb Calorimetry to Determine the Resonance Energy of Benzene
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Ammonia, Anhydrous Ama
AMMONIA, ANHYDROUS AMA CAUTIONARY RESPONSE INFORMATION 4. FIRE HAZARDS 7. SHIPPING INFORMATION 4.1 Flash Point: 7.1 Grades of Purity: Commercial, industrial, Common Synonyms Liquefied compressed Colorless Ammonia odor Not flammable under conditions likely to refrigeration, electronic, and metaflurgical Liquid ammonia gas be encountered grades all have purity greater than 99.5% 4.2 Flammable Limits in Air: 15.50%- 7.2 Storage Temperature: Ambient for pressurized 27.00% ammonia; low temperature for ammonia at Floats and boils on water. Poisonous, visible vapor cloud is produced. 4.3 Fire Extinguishing Agents: Stop flow of atmospheric pressure Avoid contact with liquid and vapor. Keep people away. gas or liquid. Let fire burn. 7.3 Inert Atmosphere: No requirement Wear goggles, self-contained breathing apparatus, and rubber overclothing (including gloves). 4.4 Fire Extinguishing Agents Not to Be 7.4 Venting: Safety relief 250 psi for ammonia Stop discharge if possible. Used: None under pressure. Pressure-vacuum for Stay upwind and use water spray to ``knock down'' vapor. 4.5 Special Hazards of Combustion ammonia at atmospheric pressure. Call fire department. Products: Not pertinent Isolate and remove discharged material. 7.5 IMO Pollution Category: Currently not available 4.6 Behavior in Fire: Not pertinent Notify local health and pollution control agencies. 7.6 Ship Type: 2 4.7 Auto Ignition Temperature: 1204°F Protect water intakes. 7.7 Barge Hull Type: 2 4.8 Electrical Hazards: Class I, Group D Combustible. 4.9 Burning Rate: 1 mm/min. Fire 8. HAZARD CLASSIFICATIONS Wear goggles, self-contained breathing apparatus, and rubber over- 4.10 Adiabatic Flame Temperature: Currently clothing (including gloves). -

HEATS of COMBUSTION of DIAMOND and of GRAPHITE by Ralph S
r---------------___........,._ . _____~ ~_ U. S. DEPARTMENT OF COMMERCE NATIONAL BUREAU OF STANDARDS RESEARCH PAPER RP1140 Part of Journal of Research of the }.{ational Bureau of Standards, Volume 21, October 1938 HEATS OF COMBUSTION OF DIAMOND AND OF GRAPHITE By Ralph S. Jessup ABSTRACT Measurements were made of the heats of combustion of one artificial graphite, two natural graphites, and two samples of diamond. The bomb calorimeter used was calibrated electrically and by means of benzoic acid. The amount of reaction in each combustion experiment was determined from the mass of carbon dioxide formed. This method of determining the amount of reaction automatically eliminates the effect of unburned carbon and of some impurities. It was found, however, that results for different samples did not agree unless the samples were purified. Two methods of purification were used: (a) heating in vacuo to 1,800° C and (b) treatment with hydrochloric and hydrofluoric acids and subsequent heating in vacuo to 200° C to remove traces of the acids. Both methods yielded graphite containing only small amounts of impurities. The mean value obtained for the heat of combustion (-/lH) of the purified graphites in oxygen at 25° C and a constant pressure of 1 atmosphere to form gaseous carbon dioxide at the same temperature and pressure was 393.396 international kilojoules per mole (44.010 g of CO2). The maximum deviation of the mean value for anyone of the purified samples from this value was 0.021 percent. The two samples of diamond, purified by treatment with aqueous hydrochloric and hydrofluoric acids and subsequently heated in vacuo to about 570° C, had average particle sizes of 2.5 I'- and 39.5 1'-, respectively, and yielded the following respective mean values for the heat of combustion (-/lH) at 25° C and 1 atmos phere, in oxygen to form gaseous carbon dioxide: 395.771 and 395.287 interna tional kilojoules per mole. -

Polar Covalent Bonds: Electronegativity
Polar Covalent Bonds: Electronegativity Covalent bonds can have ionic character These are polar covalent bonds Bonding electrons attracted more strongly by one atom than by the other Electron distribution between atoms is not symmetrical Bond Polarity and Electronegativity Symmetrical Covalent Bonds Polar Covalent Bonds C – C + - C – H C – O (non-polar) (polar) Electronegativity (EN): intrinsic ability of an atom to attract the shared electrons in a covalent bond Inductive Effect: shifting of sigma bonded electrons in resppygonse to nearby electronegative atom The Periodic Table and Electronegativity C – H C - Br and C - I (non-pol)lar) (po l)lar) Bond Polarity and Inductive Effect Nonpolar Covalent Bonds: atoms with similar EN Polar Covalent Bonds: Difference in EN of atoms < 2 Ionic Bonds: Difference in EN > 2 C–H bonds, relatively nonpolar C-O, C-X bonds (more electronegative elements) are polar Bonding electrons shift toward electronegative atom C acqqppuires partial positive char g,ge, + Electronegative atom acquires partial negative charge, - Inductive effect: shifting of electrons in a bond in response to EN of nearby atoms Electrostatic Potential Maps Electrostatic potential maps show calculated charge distributions Colors indicate electron- rich (red) and electron- poor (blue ) reg ions Arrows indicate direction of bond polarity Polar Covalent Bonds: Net Dipole Moments Molecules as a whole are often polar from vector summation of individual bond polarities and lone-pair contributions Strongly polar substances soluble in polar solvents like water; nonpolar substances are insoluble in water. Dipole moment ( ) - Net molecular polarity, due to difference in summed charges - magnitude of charge Q at end of molecular dipole times distance r between charges = Q r, in debyes (D), 1 D = 3.336 1030 coulomb meter length of an average covalent bond, the dipole moment would be 1.60 1029 Cm, or 4.80 D. -

Heterocycles 2 Daniel Palleros
Heterocycles 2 Daniel Palleros Heterocycles 1. Structures 2. Aromaticity and Basicity 2.1 Pyrrole 2.2 Imidazole 2.3 Pyridine 2.4 Pyrimidine 2.5 Purine 3. Π-excessive and Π-deficient Heterocycles 4. Electrophilic Aromatic Substitution 5. Oxidation-Reduction 6. DNA and RNA Bases 7. Tautomers 8. H-bond Formation 9. Absorption of UV Radiation 10. Reactions and Mutations Heterocycles 3 Daniel Palleros Heterocycles Heterocycles are cyclic compounds in which one or more atoms of the ring are heteroatoms: O, N, S, P, etc. They are present in many biologically important molecules such as amino acids, nucleic acids and hormones. They are also indispensable components of pharmaceuticals and therapeutic drugs. Caffeine, sildenafil (the active ingredient in Viagra), acyclovir (an antiviral agent), clopidogrel (an antiplatelet agent) and nicotine, they all have heterocyclic systems. O CH3 N HN O O N O CH 3 N H3C N N HN N OH O S O H N N N 2 N O N N O CH3 N CH3 caffeine sildenafil acyclovir Cl S N CH3 N N H COOCH3 nicotine (S)-clopidogrel Here we will discuss the chemistry of this important group of compounds beginning with the simplest rings and continuing to more complex systems such as those present in nucleic acids. Heterocycles 4 Daniel Palleros 1. Structures Some of the most important heterocycles are shown below. Note that they have five or six-membered rings such as pyrrole and pyridine or polycyclic ring systems such as quinoline and purine. Imidazole, pyrimidine and purine play a very important role in the chemistry of nucleic acids and are highlighted. -
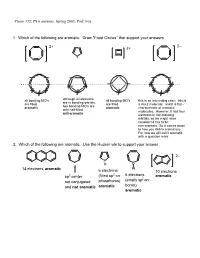
PS 6 Answers, Spring 2003, Prof
Chem 332, PS 6 answers, Spring 2003, Prof. Fox 1. Which of the following are aromatic. Draw "Frost Circles" that support your answers 2– 2+ 2+ + all bonding MO's although all electrons all bonding MO's this is an interesting case: this is are in bonding orbitals, are filled are filled a 4n+2 molecule, and it is flat-- two bonding MO's are aromatic aromatic characteristic of aromatic only half-filled molecules. However, it has four anti-aromatic electrons in non-bonding orbitals, so we might have considered this to be non-aromatic. So it comes down to how you define aromaticity. For now we will call it aromatic with a question mark. 2. Which of the following are aromatic. Use the Huckel rule to support your answer 2– P H B 14 electrons, aromatic H 6 electrons 10 electrons 2 6 electrons sp3 center (filled sp on aromatic (empty sp2 on not conjugated phosphorus) boron) and not aromatic aromatic aromatic 3. Compound 1 has an unusually large dipole moment for an organic hydrocarbon. Explain why (consider resonance structures for the central double bond) We need to remember that for any double bond compound, we can draw two polar resonance structure. Normally, these resonance structures are unimportant unimportant unimportant However, for 1 the story is different because there is a polar resonance form that has two aromatic components. In other words, 'breaking' the double bond gives rise to aromaticity, and therefore the polar resonance structure is important and leads to the observed dipole. dipole 1 8 e– 4 e– 6 e– 6 e– anti- anti- aromatic aromatic aromatic aromatic important resonance unimportant resonance structure structure 5. -

Thermochemistry of Heteroatomic Compounds:Calculation of the Heat
Open Journal of Physical Chemistry, 2011, 1, 1-5 doi:10.4236/ojpc.2011.11001 Published Online May 2011 (http://www.SciRP.org/journal/ojpc) Thermochemistry of Heteroatomic Compounds: Calculation of the heat of Combustion and the heat of Formation of some Bioorganic Molecules with Different Hydrophenanthrene Rows Vitaly V. Ovchinnikov Tupolev Kazan State Technical University, St- K. Marks 10, 420111 Kazan, Russian Federation E-mail: [email protected] Received March 23rd, 2011; revised April 10th, 2011; accepted May 10th, 2011. Abstract On the basis of the known experimental heats of combustions of the seventeen alkanes in condensed state, the general equation comb HifNg has been deduced, in which i and f are correlation coefficients, N and g are a numbers of valence electrons and lone electron pairs of heteroatoms in molecule. The pre- sented dependence has been used for the calculation of the heats of combustion of thirteen organic molecules with biochemical properties: holestan, cholesterol, methyl-cholesterol, ergosterol, vitamin-D2, estradiol, an- drostenone, testosterone, androstanedione, morphine, morphinanone, codeine and pentasozine. It is noted that good convergence was obtained within the limits of errors of thermochemical experiments known in the literature and calculations of the heats of combustion for some of them were conducted. With the application of Hess law and the heats of vaporization vap H , which has been calculated with the use of a topological 1 s solvation index of the first order x , the heats of formation f H for condensed and gaseous phases were calculated for the listed bioorganic molecules. Keywords: Alkanes, Biochemical Molecules, the Heat of Combustion, Heat of Formation, Heat of Vaporiza- tion, Topological Solvation Index 1. -

Structure of Benzene, Orbital Picture, Resonance in Benzene, Aromatic Characters, Huckel’S Rule B
Dr.Mrityunjay Banerjee, IPT, Salipur B PHARM 3rd SEMESTER BP301T PHARMACEUTICAL ORGANIC CHEMISTRY –II UNIT I UNIT I Benzene and its derivatives 10 Hours A. Analytical, synthetic and other evidences in the derivation of structure of benzene, Orbital picture, resonance in benzene, aromatic characters, Huckel’s rule B. Reactions of benzene - nitration, sulphonation, halogenationreactivity, Friedelcrafts alkylation- reactivity, limitations, Friedelcrafts acylation. C. Substituents, effect of substituents on reactivity and orientation of mono substituted benzene compounds towards electrophilic substitution reaction D. Structure and uses of DDT, Saccharin, BHC and Chloramine Benzene and its Derivatives Chemists have found it useful to divide all organic compounds into two broad classes: aliphatic compounds and aromatic compounds. The original meanings of the words "aliphatic" (fatty) and "aromatic" (fragrant/ pleasant smell). Aromatic compounds are benzene and compounds that resemble benzene in chemical behavior. Aromatic properties are those properties of benzene that distinguish it from aliphatic hydrocarbons. Benzene: A liquid that smells like gasoline Boils at 80°C & Freezes at 5.5°C It was formerly used to decaffeinate coffee and component of many consumer products, such as paint strippers, rubber cements, and home dry-cleaning spot removers. A precursor in the production of plastics (such as Styrofoam and nylon), drugs, detergents, synthetic rubber, pesticides, and dyes. It is used as a solvent in cleaning and maintaining printing equipment and for adhesives such as those used to attach soles to shoes. Benzene is a natural constituent of petroleum products, but because it is a known carcinogen, its use as an additive in gasoline is now limited. In 1970s it was associated with leukemia deaths. -

Thermal Properties of Petroleum Products
UNITED STATES DEPARTMENT OF COMMERCE BUREAU OF STANDARDS THERMAL PROPERTIES OF PETROLEUM PRODUCTS MISCELLANEOUS PUBLICATION OF THE BUREAU OF STANDARDS, No. 97 UNITED STATES DEPARTMENT OF COMMERCE R. P. LAMONT, Secretary BUREAU OF STANDARDS GEORGE K. BURGESS, Director MISCELLANEOUS PUBLICATION No. 97 THERMAL PROPERTIES OF PETROLEUM PRODUCTS NOVEMBER 9, 1929 UNITED STATES GOVERNMENT PRINTING OFFICE WASHINGTON : 1929 F<ir isale by tfttf^uperintendent of Dotmrtients, Washington, D. C. - - - Price IS cants THERMAL PROPERTIES OF PETROLEUM PRODUCTS By C. S. Cragoe ABSTRACT Various thermal properties of petroleum products are given in numerous tables which embody the results of a critical study of the data in the literature, together with unpublished data obtained at the Bureau of Standards. The tables contain what appear to be the most reliable values at present available. The experimental basis for each table, and the agreement of the tabulated values with experimental results, are given. Accompanying each table is a statement regarding the esti- mated accuracy of the data and a practical example of the use of the data. The tables have been prepared in forms convenient for use in engineering. CONTENTS Page I. Introduction 1 II. Fundamental units and constants 2 III. Thermal expansion t 4 1. Thermal expansion of petroleum asphalts and fluxes 6 2. Thermal expansion of volatile petroleum liquids 8 3. Thermal expansion of gasoline-benzol mixtures 10 IV. Heats of combustion : 14 1. Heats of combustion of crude oils, fuel oils, and kerosenes 16 2. Heats of combustion of volatile petroleum products 18 3. Heats of combustion of gasoline-benzol mixtures 20 V. -

Chapter Two Properties of Ammonia
Chapter Two Properties of Ammonia Physical Properties General Anhydrous ammonia exists as either a colorless gas, colorless liquid, or white solid, depending on its pressure and temperature. In nearly all commonly encountered situations, it exists as either a liquid or a gas. The gas is less dense than air and the liquid is less dense than water at standard conditions. Ammonia gas (vapor) diffuses readily in air and the liquid is highly soluble in water with an accompanying release of heat. Ammonia exhibits classical saturation relationships whereby pressure and temperature are directly related so long as both the vapor and liquid phase are present. It does have a critical pressure and temperature. At atmospheric pressure, a closed container of ammonia vapor and liquid will be in equilibrium at a temperature of –28°F [–33°C]. It should be noted however that if liquid ammonia is spilled or released to the atmosphere at normal temperatures, the resultant pool of boiling liquid will be significantly colder than –28°F due to the law of partial pressures (the partial pressure of the ammonia vapor in the air near the liquid surface will be less than atmospheric pressure). The following table provides some of the important physical properties of ammonia. TABLE 2-1 Physical Properties of Ammonia Property Condition Value (IP) Value (SI) Molecular Weight 17.03 17.03 Color None None Physical State Room Temp Gas Gas Freezing Point P=1 atm –108°F –78°C Boiling Point P=1 atm –28.1°F –33.3°C Critical Pressure 1657 psia 11,410 kPa Critical Temp 271°F -
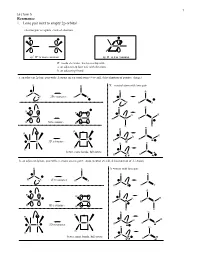
Resonance 1. Lone Pair Next to Empty 2P Orbital
1 Lecture 5 Resonance 1. Lone pair next to empty 2p orbital electron pair acceptors - lack of electrons C C CC sp2 R+ is more common sp R+ is less common R+ needs electrons, has to overlap with a. an adjacent 2p lone pair with electrons b. an adjacent pi bond a. an adjacent 2p lone pair with electrons on a neutral atom (+ overall, delocalization of positive charge) R R X = neutral atom with lone pair R R C C R X 2D resonance R X C C R X R X R R R R C R C R CX CX R N R N R 3D resonance R R R R R R R C R C R CX R O CX 3D resonance R O R R R R C better, more bonds, full octets C R F R F b. an adjacent 2p lone pair with electrons on a negative atom (neutral overall, delocalization of electrons) R R X =anion with lone pair R R C C R X 2D resonance R X C C R X R X R R R R C R C R CX CX R C R C R 3D resonance R R R R R R R C R C R CX R N CX 3D resonance R N R R R R C C better, more bonds, full octets R O R O 2 Lecture 5 Problem 1 – All of the following examples demonstrate delocalization of a lone pair of electrons into an empty 2p orbital. Usually in organic chemistry this is a carbocation site, but not always. -

Physics of Resonating Valence Bond Spin Liquids Julia Saskia Wildeboer Washington University in St
Washington University in St. Louis Washington University Open Scholarship All Theses and Dissertations (ETDs) Summer 8-13-2013 Physics of Resonating Valence Bond Spin Liquids Julia Saskia Wildeboer Washington University in St. Louis Follow this and additional works at: https://openscholarship.wustl.edu/etd Recommended Citation Wildeboer, Julia Saskia, "Physics of Resonating Valence Bond Spin Liquids" (2013). All Theses and Dissertations (ETDs). 1163. https://openscholarship.wustl.edu/etd/1163 This Dissertation is brought to you for free and open access by Washington University Open Scholarship. It has been accepted for inclusion in All Theses and Dissertations (ETDs) by an authorized administrator of Washington University Open Scholarship. For more information, please contact [email protected]. WASHINGTON UNIVERSITY IN ST. LOUIS Department of Physics Dissertation Examination Committee: Alexander Seidel, Chair Zohar Nussinov Michael C. Ogilvie Jung-Tsung Shen Xiang Tang Li Yang Physics of Resonating Valence Bond Spin Liquids by Julia Saskia Wildeboer A dissertation presented to the Graduate School of Arts and Sciences of Washington University in partial fulfillment of the requirements for the degree of Doctor of Philosophy August 2013 St. Louis, Missouri TABLE OF CONTENTS Page LIST OF FIGURES ................................ v ACKNOWLEDGMENTS ............................. ix DEDICATION ................................... ix ABSTRACT .................................... xi 1 Introduction .................................. 1 1.1 Landau’s principle of symmetry breaking and topological order .... 2 1.2 From quantum dimer models to spin models .............. 5 1.2.1 The Rokhsar-Kivelson (RK) point ................ 12 1.3 QDM phase diagrams ........................... 14 1.4 Z2 quantum spin liquid and other topological phases .......... 15 1.4.1 Z2 RVB liquid ........................... 16 1.4.2 U(1) critical RVB liquid .................... -

Physical Organic Chemistry
PHYSICAL ORGANIC CHEMISTRY Yu-Tai Tao (陶雨台) Tel: (02)27898580 E-mail: [email protected] Website:http://www.sinica.edu.tw/~ytt Textbook: “Perspective on Structure and Mechanism in Organic Chemistry” by F. A. Corroll, 1998, Brooks/Cole Publishing Company References: 1. “Modern Physical Organic Chemistry” by E. V. Anslyn and D. A. Dougherty, 2005, University Science Books. Grading: One midterm (45%) one final exam (45%) and 4 quizzes (10%) homeworks Chap.1 Review of Concepts in Organic Chemistry § Quantum number and atomic orbitals Atomic orbital wavefunctions are associated with four quantum numbers: principle q. n. (n=1,2,3), azimuthal q.n. (m= 0,1,2,3 or s,p,d,f,..magnetic q. n. (for p, -1, 0, 1; for d, -2, -1, 0, 1, 2. electron spin q. n. =1/2, -1/2. § Molecular dimensions Atomic radius ionic radius, ri:size of electron cloud around an ion. covalent radius, rc:half of the distance between two atoms of same element bond to each other. van der Waal radius, rvdw:the effective size of atomic cloud around a covalently bonded atoms. - Cl Cl2 CH3Cl Bond length measures the distance between nucleus (or the local centers of electron density). Bond angle measures the angle between lines connecting different nucleus. Molecular volume and surface area can be the sum of atomic volume (or group volume) and surface area. Principle of additivity (group increment) Physical basis of additivity law: the forces between atoms in the same molecule or different molecules are very “short range”. Theoretical determination of molecular size:depending on the boundary condition.