Functional Morphology of the Radialis Muscle in Shark Tails
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
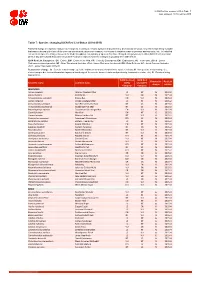
Table 7: Species Changing IUCN Red List Status (2014-2015)
IUCN Red List version 2015.4: Table 7 Last Updated: 19 November 2015 Table 7: Species changing IUCN Red List Status (2014-2015) Published listings of a species' status may change for a variety of reasons (genuine improvement or deterioration in status; new information being available that was not known at the time of the previous assessment; taxonomic changes; corrections to mistakes made in previous assessments, etc. To help Red List users interpret the changes between the Red List updates, a summary of species that have changed category between 2014 (IUCN Red List version 2014.3) and 2015 (IUCN Red List version 2015-4) and the reasons for these changes is provided in the table below. IUCN Red List Categories: EX - Extinct, EW - Extinct in the Wild, CR - Critically Endangered, EN - Endangered, VU - Vulnerable, LR/cd - Lower Risk/conservation dependent, NT - Near Threatened (includes LR/nt - Lower Risk/near threatened), DD - Data Deficient, LC - Least Concern (includes LR/lc - Lower Risk, least concern). Reasons for change: G - Genuine status change (genuine improvement or deterioration in the species' status); N - Non-genuine status change (i.e., status changes due to new information, improved knowledge of the criteria, incorrect data used previously, taxonomic revision, etc.); E - Previous listing was an Error. IUCN Red List IUCN Red Reason for Red List Scientific name Common name (2014) List (2015) change version Category Category MAMMALS Aonyx capensis African Clawless Otter LC NT N 2015-2 Ailurus fulgens Red Panda VU EN N 2015-4 -

Shark Cartilage, Cancer and the Growing Threat of Pseudoscience
[CANCER RESEARCH 64, 8485–8491, December 1, 2004] Review Shark Cartilage, Cancer and the Growing Threat of Pseudoscience Gary K. Ostrander,1 Keith C. Cheng,2 Jeffrey C. Wolf,3 and Marilyn J. Wolfe3 1Department of Biology and Department of Comparative Medicine, Johns Hopkins University, Baltimore, Maryland; 2Jake Gittlen Cancer Research Institute, Penn State College of Medicine, Hershey, Pennsylvania; and 3Registry of Tumors in Lower Animals, Experimental Pathology Laboratories, Inc., Sterling, Virginia Abstract primary justification for using crude shark cartilage extracts to treat cancer is based on the misconception that sharks do not, or infre- The promotion of crude shark cartilage extracts as a cure for cancer quently, develop cancer. Other justifications represent overextensions has contributed to at least two significant negative outcomes: a dramatic of experimental observations: concentrated extracts of cartilage can decline in shark populations and a diversion of patients from effective cancer treatments. An alleged lack of cancer in sharks constitutes a key inhibit tumor vessel formation and tumor invasions (e.g., refs. 2–5). justification for its use. Herein, both malignant and benign neoplasms of No available data or arguments support the medicinal use of crude sharks and their relatives are described, including previously unreported shark extracts to treat cancer (6). cases from the Registry of Tumors in Lower Animals, and two sharks with The claims that sharks do not, or rarely, get cancer was originally two cancers each. Additional justifications for using shark cartilage are argued by I. William Lane in a book entitled “Sharks Don’t Get illogical extensions of the finding of antiangiogenic and anti-invasive Cancer” in 1992 (7), publicized in “60 Minutes” television segments substances in cartilage. -

Sharks in Crisis: a Call to Action for the Mediterranean
REPORT 2019 SHARKS IN CRISIS: A CALL TO ACTION FOR THE MEDITERRANEAN WWF Sharks in the Mediterranean 2019 | 1 fp SECTION 1 ACKNOWLEDGEMENTS Written and edited by WWF Mediterranean Marine Initiative / Evan Jeffries (www.swim2birds.co.uk), based on data contained in: Bartolí, A., Polti, S., Niedermüller, S.K. & García, R. 2018. Sharks in the Mediterranean: A review of the literature on the current state of scientific knowledge, conservation measures and management policies and instruments. Design by Catherine Perry (www.swim2birds.co.uk) Front cover photo: Blue shark (Prionace glauca) © Joost van Uffelen / WWF References and sources are available online at www.wwfmmi.org Published in July 2019 by WWF – World Wide Fund For Nature Any reproduction in full or in part must mention the title and credit the WWF Mediterranean Marine Initiative as the copyright owner. © Text 2019 WWF. All rights reserved. Our thanks go to the following people for their invaluable comments and contributions to this report: Fabrizio Serena, Monica Barone, Adi Barash (M.E.C.O.), Ioannis Giovos (iSea), Pamela Mason (SharkLab Malta), Ali Hood (Sharktrust), Matthieu Lapinksi (AILERONS association), Sandrine Polti, Alex Bartoli, Raul Garcia, Alessandro Buzzi, Giulia Prato, Jose Luis Garcia Varas, Ayse Oruc, Danijel Kanski, Antigoni Foutsi, Théa Jacob, Sofiane Mahjoub, Sarah Fagnani, Heike Zidowitz, Philipp Kanstinger, Andy Cornish and Marco Costantini. Special acknowledgements go to WWF-Spain for funding this report. KEY CONTACTS Giuseppe Di Carlo Director WWF Mediterranean Marine Initiative Email: [email protected] Simone Niedermueller Mediterranean Shark expert Email: [email protected] Stefania Campogianni Communications manager WWF Mediterranean Marine Initiative Email: [email protected] WWF is one of the world’s largest and most respected independent conservation organizations, with more than 5 million supporters and a global network active in over 100 countries. -

Fishery Bulletin/U S Dept of Commerce National Oceanic
ASPECTS OF THE BIOWGY OF TWO SCYLIORHINID SHARKS, APRISTURUS BRUNNEUS AND PARMATURUS XANIURUS, FROM THE UPPER CONTINENTAL SWPE OFF SOUTHERN CALIFORNIA JEFFREY N. CROSSI ABSTRACf The distribution. abundance, reproductive cycle. and food habits of two scyliorhinid sharks are discussed. Catsharks occurred on 87% of 71 longline sets and in 6% of 48 trawls. Longline catches were stratified by habitat into banks (hard substrate) and mud (soft substrate). ApriBtu'l"lUl brllnnell.8 occurred more frequently on mud sets than on bank sets. but its abundance was similar in both habitats. Pa'MIlaturus :l'a·t/.iu~ oC<.'l1rred equally frequently on mud and bank sets, but it was more abundant on bank sets. Catches of both species consisted of adults and adolescents; juveniles were rare or absent. Historical collections suggest that juveniles are mesopelagic. Male P. zaniurull matured ata smaller size than male A. brrt1meus. Females of both species matured at about the same size and fecundity increased with female size. The proportion of body weight devoted to gonads and maximum oocyte size were greater among P. 2:a1liurus, but fecundity and the proportion of females carrying egg cases were greater among A. brun'IU.l1ts. Seasonal changes in gonadal develop ment were not well defined for either species. Members of both populations may have been reproduc tively active throughout the year. The diets of both species comprised. in order of importance, crustaceans, teleosts, and squids. Most prey consumed were pelagic; however, it is not known where in the water column the catsharks obtained their prey. The Scyliorhinidae is the largest family of living ParmatWr1"S Xa11Jiu1'US Gilbert, the filetail cat sharks with about 94 valid species (Nelson 1984). -

Spiny Dogfish.Pdf
Memorandum of Understanding on the Conservation of Migratory Sharks SPINYSILKY DOGFISH SHARK AIGUILLATREQUIN COMMUNSOYEUX TIBURONMIELGA/GALLUDO SEDOSO Fact Sheet TiburonesTiburones martillomartillo Spiny Dogfish Squalus acanthias SPINY DOGFISH Class: Chondrichthyes Order: Squaliformes Family: Squalidae Species: Squalus acanthias Illustration: © Marc Dando Sharks MOU Species Fact Sheet Sharks MOU Species Fact Sheet SPINY DOGFISH SPINY DOGFISH © Shark MOU Advisory Committee This fact sheet was produced by the Advisory Committee of the Memorandum of Understanding on the Conservation of Migratory Sharks (Sharks MOU). For further information contact: John Carlson, Ph.D. Research Fish Biologist, NOAA Fisheries Service-Southeast Fisheries Science Center Panama City, [email protected] 1 Sharks MOU Species Fact Sheet SPINY DOGFISH 1. Biology Spiny Dogfish (Squalus acanthias), also known as Picked Dogfish or Spurdog, is a demersal shark that has a maximum length of 125 cm in the North Atlantic. It occurs mostly in shelf seas, from coastal habitats to the shelf edge, but can occur to depths of 900 m. They aggregate by size and sex, and are migratory in regional seas, although very occasional transatlantic movements have been reported. Spiny Dogfish are long lived (ca. 50–60 years) and have slow growth rates. Females mature at a length of 75-85 cm, produce up to 21 pups and gestation lasts two years (ICES 2017). Published studies on S. acanthias from the North Pacific relate to Squalus suckleyi (see Ebert et al. 2010). 2. Distribution Spiny Dogfish is distributed in both northern and southern temperate and boreal waters, but the species is listed on the MOU for the northern hemisphere populations only. -

Identification Guide to the Deep-Sea Cartilaginous Fishes Of
Identification guide to the deep–sea cartilaginous fishes of the Southeastern Atlantic Ocean FAO. 2015. Identification guide to the deep–sea cartilaginous fishes of the Southeastern Atlantic Ocean. FishFinder Programme, by Ebert, D.A. and Mostarda, E., Rome, Italy. Supervision: Merete Tandstad, Jessica Sanders (FAO, Rome) Technical editor: Edoardo Mostarda (FAO, Rome) Colour illustrations, cover and graphic design: Emanuela D’Antoni (FAO, Rome) This guide was prepared under the “FAO Deep–sea Fisheries Programme” thanks to a generous funding from the Government of Norway (Support to the implementation of the International Guidelines on the Management of Deep-Sea Fisheries in the High Seas project) for the purpose of assisting states, institutions, the fishing industry and RFMO/As in the implementation of FAO International Guidelines for the Management of Deep-sea Fisheries in the High Seas. It was developed in close collaboration with the FishFinder Programme of the Marine and Inland Fisheries Branch, Fisheries Department, Food and Agriculture Organization of the United Nations (FAO). The present guide covers the deep–sea Southeastern Atlantic Ocean and that portion of Southwestern Indian Ocean from 18°42’E to 30°00’E (FAO Fishing Area 47). It includes a selection of cartilaginous fish species of major, moderate and minor importance to fisheries as well as those of doubtful or potential use to fisheries. It also covers those little known species that may be of research, educational, and ecological importance. In this region, the deep–sea chondrichthyan fauna is currently represented by 50 shark, 20 batoid and 8 chimaera species. This guide includes full species accounts for 37 shark, 9 batoid and 4 chimaera species selected as being the more difficult to identify and/or commonly caught. -

Efficacy of 2 Common Bait Types in Reducing Bycatch of Coastal Sharks 191
189 National Marine Fisheries Service Fishery Bulletin First U.S. Commissioner established in 1881 of Fisheries and founder NOAA of Fishery Bulletin Abstract—A recent study determined Efficacy of 2 common bait types in reducing that when simultaneously exposed to 2 different commonly used baits, certain bycatch of coastal sharks on bottom longline shark species demonstrate preferences for a specific bait on bottom longlines. gear in the absence of choice To further investigate the value of bait type to reduce shark bycatch, we con- William B. Driggers III (contact author)1 ducted single- bait- type bottom longline Kristin M. Hannan2 sets with standardized gear baited with either mackerel or squid. For 4 of Email address for contact author: [email protected] the 5 shark species captured, there was no significant difference in catch rates 1 with bait type. However, catch rates of Mississippi Laboratories Atlantic sharpnose sharks (Rhizopri- Southeast Fisheries Science Center onodon terraenovae) were significantly National Marine Fisheries Service, NOAA higher on mackerel- baited hooks. Our 3209 Frederic Street results indicate that the use of squid Pascagoula, Mississippi 39567-4112 as bait can reduce the catch of at least 2 Riverside Technology Inc. one shark species in the northern Gulf Mississippi Laboratories of Mexico while not reducing the catch Southeast Fisheries Science Center of a targeted species, in this case, the National Marine Fisheries Service, NOAA red snapper (Lutjanus campechanus). 3209 Frederic Street However, because some protected spe- Pascagoula, Mississippi 39567-4112 cies, most notably sea turtles, have been shown to have higher catch rates on squid- baited hooks, it is necessary to assess the effect of a specific bait across all taxa directly or indirectly affected by a particular gear type before adopt- Globally, shark populations are widely et al. -

Cites Proposal 18 Spiny Dogfish Shark
CITES PROPOSAL 18 SPINY DOGFISH SHARK www.pewenvironment.org/cites Andy Murch/SeaPics.com Biological vulnerability to over-exploitation SPINY DOGFISH SHARK (Squalus acanthias) • Slow to reach maturity: Females: Proposed by Sweden on behalf of Appendix II European Union Member States 6 years, Northwest Atlantic listing and Palau 15 years, Northeast Atlantic Critically Endangered in Northeast 23 to 32 years, Northeast Pacific Males: IUCN Atlantic Red List status Endangered in Northwest Atlantic 10 years, Northwest Atlantic 2 14 years, Northeast Pacific Vulnerable globally • Low reproductive capacity, with only one to 20 pups per litter.3 RECOMMENDATION: SUPPORT • Long lives; some stocks are thought to have • The Pew Environment Group applauds the individuals that live up to 100 years.4 submission of this proposal and urges CITES Parties to support it. • Very long gestation period of 18 to 22 months.5 • Spiny dogfish are in the U.N. Food and Agriculture Organisation’s lowest productivity category and are Spiny dogfish fisheries and trade extremely vulnerable to over-exploitation because The spiny dogfish is a high-value commercial species of their slowness to reach reproductive maturity, experiencing over-exploitation in target and bycatch lengthy gestation and small litters.1 fisheries. The fish are caught in bottom trawls, gillnets and line gear, and by rod and reel. Exploitation is • A strong international demand for spiny dogfish fueled primarily by strong international demand meat and other products has fueled unsustainable for its meat, often sold as rock salmon, rock eel or harvest of this vulnerable species. flake. The European Union is a major importer of the • Fisheries records and stock assessment information meat, although fins and other spiny dogfish products have revealed steep declines in reproductive are traded internationally as well.6 This species is biomass of spiny dogfish around the globe. -

Conclusions GRS 1 3 5 6 8 9 10 12 16 18 20 21 31 35 36 38 40 42 45 GREENLAND SEA
a a b c d e a) b) c) d) e) Lynghammar University of Tromsø, Norway, A., Christiansen University of Washington,, J. USAS., Gallucci Murmansk Marine, V. BiologicalF., Karamushko Institute, Russia California, O. V., AcademyMecklenburg of Sciences, USA, C. Natural W. History& Møller Museum ,of P. Denmark R. Contact: [email protected] introduction The sea ice cover decreases and human activity increases in Arctic waters. Fisheries (by-catch issues), shipping and petroleum exploita- tion (pollution issues) make it imperative to establish biological base- OCCURRENCE OF lines for the marine fishes inhabiting the Arctic Ocean and adjacent seas (AOAS). As a first step towards credible conservation actions for the Arctic marine fish faunae, we examine the species-richness of chondrich- thyan fishes (class Chondrichthyes) pertaining to 16 regions within the AOAS: chimaeras, sharks and skates. CHONDRICHTHYAN materials and methods • Voucher specimens from Natural History Collections IN THE ARCTIC OCEAN • Annotated checklists (see selected references) • The CAFF Database on Arctic marine fishes (Christiansen et al., in AND ADJACENT SEAS progress) FISHES Only presence and absence data are considered, as reliable abundance data lack for most species. Occurrences known only from floating or beach-cast carcasses, such as Pacific sleeper shark (no. 17) and Alaska skate (no. 29) in the Chukchi Sea, are not considered conclusive evidence of presence and are not included. CHIMAERIFORMES HEXANCHIFORMES RAJIFORMES Chimaeridae - ratfishes Chlamydoselachidae -

European Shark Guide
The European Shark Guide If you are heading for a European coastline this summer, the chances are you will be sharing the sea with some fascinating, but increasingly rare fish. That’s not meant to alarm you. The idea that sharks pose a serious danger to humans is a myth. The threat to sharks The fact is that this extraordinary group of fish is seriously threatened by human activities. European sharks are judged more at risk of extinction than those in most other assessed regions of the world. Europeans have a taste for shark meat that has driven several species to the brink. The shark’s most famous feature – the fin – is also at the heart of the threat to sharks. You can make a difference The EU banned shark finning in 2003, (please see page 9) but loopholes in the regulation seriously hamper enforcement. MEPs called on the European Commission to strengthen the shark finning ban nearly four years ago. In the coming months, the process for amending this critical regulation will finally begin in earnest. The Shark Alliance, a coalition of NGOs dedicated to restoring and conserving shark populations, has produced this fact-packed guide to give you some insight in to the amazing world of sharks, and help MEPs to conserve these remarkable but imperilled fish. All information was taken and adapted from Shark Alert by Sonja Fordham and other Shark Alliance publications. 1 Now Fas cin ating shark People evolve facts we think you ’ll like to know: Dinosaurs die Sharks, in some form, have roamed our seas 100 million years ago for more than 400 million years, which means their ancestors inhabited the earth for nearly 200 million years before dinosaurs. -

Centrophorus Squamosus
Published Date: 1 March 2019 Leafscale Gulper Shark, Centrophorus squamosus Report Card Undefined Stock assessment IUCN Red List IUCN Red List Refer to Global Australian Global Vulnerable Assessment Assessment Assessment Assessors White, W.T. & Graham, K.J. Sensitive to fishing pressure with some catches in Australia but Report Card Remarks uncertainty over stock status Summary The Leafscale Gulper Shark is a deepwater dogfish with a global distribution. Its flesh and livers are marketed in varying quantities over much of the species' range. Most targeted fishing pressure is in the northeast Atlantic where it is an important component of deepwater fisheries and declines of Source: CSIRO National Fish Collection. License: CC BY Attribution >80% have been reported. Catches of the species off Australia are relatively low and do not represent a significant component of deepwater catches. However, significant declines in closely related species in southeast Australia led to management measures to promote recovery of deepwater dogfish populations, such as catch limits and a ban on trawling below 700 m. The Leafscale Gulper Shark has intrinsically very low productivity, evident in significant population declines where it is heavily fished. Therefore, the species is assessed as globally Vulnerable (IUCN) and in Australia, Undefined Stock (SAFS) because the information is uncertain and insufficient to assess the Australian fish stock status. Distribution The Leafscale Gulper Shark occurs sporadically in the eastern Atlantic (Iceland to South Africa), in the Indian Ocean (South Africa and the Aldabra Islands), and the western Pacific (Japan, Taiwan, Philippines, Indonesia, New Zealand and Australia); a recent capture off the Galapagos Island extended the range to the eastern central Pacific (Acuna-Marrero et al. -

5 November 1997
An identification guide for deepwater shark species Di Tracey and Peter Shearer National Institute of Water and Atmospheric Research August 2002 Contents Page Baxter’s dogfish (Etmopterus baxteri) 2 Catsharks (Apristurus spp.) 3 Leafscale gulper shark (Centrophorus squamosus) 4 Longnose velvet dogfish (Centroscymnus crepidater) 5 Lucifer dogfish (Etmopterus lucifer) 6 Northern spiny dogfish (Squalus mitsukurii) 7 Owston’s dogfish (Centroscymnus owstoni) 8 Plunket’s shark (Centroscymnus plunketi) 9 Portuguese dogfish (Centroscymnus coelolepis) 10 Prickly dogfish (Oxynotus bruniensis) 11 Seal shark (Dalatias licha) 12 Shovelnose dogfish (Deania calcea) 13 Spiny dogfish (Squalus acanthias) 14 NIWA Guide to Some Common New Zealand Deepwater Sharks – August 2002 Introduction The aim of this guide is to provide clear and concise identification sheets for deepwater shark species for future use by scientific observers and the fishing industry. This guide is an updated version of the Deepwater Shark Reference Guide sheets already prepared by NIWA. The shark species are presented alphabetically by common name. Layout for each species is consistent throughout the document and includes the following: • common name, scientific name, and species code • colour photograph and line drawing for each species • information on the depth distribution of the species • diagnostic features – outlines the key characteristics used to identify the species • maximum lengths that are attained • colour photograph and line drawing of the underside of head for some species These sheets can be substituted for the sheets already included in the revised Observer Manual. Enclosed is a CD ROM containing the shark guide as an interactive PDF document, an Acrobat Reader installer, and a word document.