Phosgene Oxime
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
![Endobj 21 0 Obj <>/Filter/Flatedecode/ID[]/Index[16 7]](https://docslib.b-cdn.net/cover/9015/endobj-21-0-obj-filter-flatedecode-id-index-16-7-69015.webp)
Endobj 21 0 Obj <>/Filter/Flatedecode/ID[]/Index[16 7]
%PDF-1.4 %âãÏÓ 16 0 obj <> endobj 21 0 obj <>/Filter/FlateDecode/ID[]/Index[16 7]/Info 15 0 R/Length 36/Prev 1529327/Root 17 0 R/Size 23/Type/XRef/W[1 2 0]>>stream hÞbbd`b`r`b`cb`>stream hÞb```f``âc```‘¨`b@ ›…£éœÌ®5zà $À Å 9 Â Ü Ì‚`.ƒlÕ ÍÄ÷ ,"† endstream endobj 17 0 obj < > endobj 18 0 obj < >>>/Rotate 0/Type/Page>> endobj 19 0 obj <>stream H‰*ä234Ò300P AsK;9—KßËÓ0QÁ%Ÿ+ À H endstream endobj 20 0 obj <>stream ÿØÿà JFIF È È ÿþ Business Office Q76ÿÛ C $.' ",#(7),01444'9=82 À@ú– ~5â¾Óµï‰V%Õ ¢¾bø‡ã?xKÄ_Ùñj:eŵÄms{24.@C†ÁÇ=ý+¼ø[©ø¯Äš$ö©`±]G †Ö;6R¤6ÕvmÜò§ØõôsØh¯– •êž ¿ñ^»áØuBïKI/ao Z>"ç†cæ|Û‡8ã¿jzògˆþ+øÓ@ÖõM"{- å°+—Xåë.å#çãå#ŽÕêi?Ål’æðƒ»¨qûHÊ‘‘ϯ·Oz ö +Ç>xÇÄ>!ÕuÍ+Äz]¶Ÿw¦y@Çs—Üy%ˆ# G-7âÄ;ZÓ|1 Yï_°&b"…OBøç=ñè3ée¢¼CRÔ¾&øvÖãT»³Ðµ»X†ù-,|ØfUï³ çŽÇ'Ž3W-|}zÿ ÛÆ’Ù[›¡È-ÑÎÎ%(y= 'ß=(Øè¯ “Åß`ðôÚÝÏ„´ûh¡·7פJ.âJcƒŒü¤‚:ž+šðïÅÏxŠþ?Mð½¬×2De ÞìF2yâ€>š¢¼'Dñ÷Šçñm—†õ¿ G¥És ²¬†äJ¬ e㨠ã$dqYÞ$ø¥¯x_UþËÕü5l&h„ÑIoz]$L‘JÔt#4ô=àº7|q¬é°ê–>Žk)”¼nº¤`°=½+sÁ?/ øRÞI"›ÄºLrFþ[£^F ·Lc=³Ï§9èk¯R òï@EPEPEPEPETº½µ³]×W0À¾²È~µ–«§_ŒÙ_ÚÜŒ•ýÌÊüœp}(JŠ¨·¶¬$+s ¹ŽB$#ÿ túGñsé Š( Š( Š( Š( Š( Š( Š( Š( Š( Š( Š( Š( Š( Š( Š( Š( Š( Š( Š( Š( Š( Š( Š( Š( Š( Š( Š( Š( Š( Š( Š( Š( Š( Š( Š( Š( Š( Š( Š( Š( Š( Š( Š( Š( Š( Š( Š( Š( Š( Š( Š( Š( Š( Š( Š( Š( Š( Š( Š( Š( Š( Š( Š( Š( Š( Š( Š( Š( çŠ9âxe@ñÈ¥]OBWç¼2Kðãâ@ÌB(¬oXÚyläóŽçc{ãÒ¿C+äoÚKI÷ºv¸‘'…¬å9Ç –_ǹö Kø¼÷$¶²ðfñ5Î-ܼÊ$1 ë’äç½|ýð_þÈñݧÚTEöøÛO™U—pÛ‘žà~ ¯qø §_Ë£jÓ šÑ¼Ï|EÕuR]´ý¦Û÷~ÐØy˜¹bžÄc-Mñ´ð÷Yʆâƒÿ ]’´þxrO xVÎÎá¦kÙ³svf}Íç>dþúu=k7ãgü“ícþØÿ èä gÍ¿ üa£ø3YÕoµ‰fQ-š¬1Åv•ƒg°?\z÷ÿ ƒZ™ñW†5;ûÍÒ¥ö¥rÍœ…FƯ@§åßbŽãÄ:äSD²Fl£R®¡•cAîü4 ¦¿=|[á]KÀ¾3ÒìÅóÊ%Ó0p~Àÿ úz'Â?hžÑ-nuÍ6ˆ¡"H¤»]öê -

Transport of Dangerous Goods
ST/SG/AC.10/1/Rev.16 (Vol.I) Recommendations on the TRANSPORT OF DANGEROUS GOODS Model Regulations Volume I Sixteenth revised edition UNITED NATIONS New York and Geneva, 2009 NOTE The designations employed and the presentation of the material in this publication do not imply the expression of any opinion whatsoever on the part of the Secretariat of the United Nations concerning the legal status of any country, territory, city or area, or of its authorities, or concerning the delimitation of its frontiers or boundaries. ST/SG/AC.10/1/Rev.16 (Vol.I) Copyright © United Nations, 2009 All rights reserved. No part of this publication may, for sales purposes, be reproduced, stored in a retrieval system or transmitted in any form or by any means, electronic, electrostatic, magnetic tape, mechanical, photocopying or otherwise, without prior permission in writing from the United Nations. UNITED NATIONS Sales No. E.09.VIII.2 ISBN 978-92-1-139136-7 (complete set of two volumes) ISSN 1014-5753 Volumes I and II not to be sold separately FOREWORD The Recommendations on the Transport of Dangerous Goods are addressed to governments and to the international organizations concerned with safety in the transport of dangerous goods. The first version, prepared by the United Nations Economic and Social Council's Committee of Experts on the Transport of Dangerous Goods, was published in 1956 (ST/ECA/43-E/CN.2/170). In response to developments in technology and the changing needs of users, they have been regularly amended and updated at succeeding sessions of the Committee of Experts pursuant to Resolution 645 G (XXIII) of 26 April 1957 of the Economic and Social Council and subsequent resolutions. -

1981-04-15 EA Plan of Development Production
United States Department of the Interior Office of the Secretary Minerals Management Service 1340 West Sixth Street Los Angeles, California 90017 OCS ENVIRONMENTAL ASSESSMENT July 8, 1982 Operator Chevron U.S.A. Inc. Plan Type Development/Production Lease OCS-P 0296 Block 34 N., 37 W. Pl atfonn Edith Date Submitted April 15, 1981 Prepared by the Office of the Deputy Minerals Manager, Field Operations, Pacific OCS Region Related Environmental Documents U. S. DEPARTMENT OF THE INTERIOR GEOLOGICAL SURVEY Environmental Impact Report - Environmental Assessment, Shell OCS Beta Unit Development (prepared jointly with agencies of the State of California, 1978) 3 Volumes Environmental Assessment, Exploration, for Lease OCS-P 0296 BUREAU OF LAND MANAGEMENT Proposed 1975 OCS Oil and Gas General Lease Sale Offshore Southern California (OCS Sale No. 35), 5 Volumes Proposed 1979 OCS Oil and Gas Lease Sale Offshore Southern California (OCS Sale No. 48), 5 Volumes Proposed 1982 OCS Oil and Gas General Lease Sale Offshore Southern California (OCS Sale No. 68), 2 Volumes u.c. Santa Cruz - BLM, Study of Marine Mammals and Seabirds of the Southern California Bight ENVIRONMENTAL ASSESSMENT CHEVRON U.S.A. INC. OPERATOR PLAN OF DEVELOPMENT/PRODUCTION, PROPOSED PLATFORM EDITH, LEASE OCS-P 0296, BETA AREA, SAN PEDRO BAY, OFFSHORE SOUTHERN CALIFORNIA Table of Contents Page I. DESCRIPTION OF THE PROPOSED ACTION ••••••••••••••••••••• 1 II. DESCRIPTION OF AFFECTED ENVIRONMENT •••••••••••••••••••• 12 III. ENVIRONMENTAL CONSEQUENCES ••••••••••••••••••••••••••••• 29 IV. ALTERNATIVES TO THE PROPOSED ACTION •••••••••••••••••••• 46 v. UNAVOIDABLE ADVERSE ENVIRONMENTAL EFFECTS •••••••••••••• 48 VI. CONTROVERSIAL ISSUES ••••••••••••••••••••••••••••••••••• 48 VII. FINDING OF NO SIGNIFICANT IMPACT (FONS!) ••••••••••••••• 51 VIII. ENVIRONMENTAL ASSESSMENT DETERMINATION ••••••••••••••••• 55 IX. -

Copyrighted Material
1 Historical Milieu 1.1 Organophosphorus Nerve Agents 2 1.2 Blister Agents 5 1.3 Sternutator Agents 11 1.4 Chemical Weapons Convention (CWC) 13 1.4.1 Schedule of Chemicals 14 1.4.2 Destruction of Chemical Weapons 14 References 16 COPYRIGHTED MATERIAL Analysis of Chemical Warfare Degradation Products, First Edition. Karolin K. Kroening, Renee N. Easter, Douglas D. Richardson, Stuart A. Willison and Joseph A. Caruso. © 2011 John Wiley & Sons, Ltd. Published 2011 by John Wiley & Sons, Ltd. 2 ANALYSIS OF CHEMICAL WARFARE DEGRADATION PRODUCTS 1.1 ORGANOPHOSPHORUS NERVE AGENTS Organophosphorus (OP) type compounds, that is, deriva- tives containing the P=O moiety, were first discovered in the 1800s when researchers were investigating useful applica- tions for insecticides/rodenticides. There are many derivatives of organophosphorus compounds, however, the OP deriva- tives that are typically known as ‘nerve agents’ were discov- ered accidentally in Germany in 1936 by a research team led by Dr. Gerhard Schrader at IG Farben [1–4]. Schrader had noticed the effects and lethality of these organophosphorus compounds towards insects and began developing a new class of insecticides. While working towards the goal of an improved insecticide, Schrader experimented with numerous phosphorus-containing compounds, leading to the discovery of the first nerve agent, Tabun (or GA) (Figure 1.1). The potency of these insecticides towards humans was not realized until there was yet another accident, which involved a Tabun spill. Schrader and coworkers began experiencing symptoms, such as miosis (constriction of the pupils of the eyes), dizziness and severe shortness of breath, with numerous effects lasting several weeks [1, 4, 5]. -

Downloads/DL Praevention/Fachwissen/Gefahrstoffe/TOXIKOLOGI SCHE BEWERTUNGEN/Bewertungen/Toxbew072-L.Pdf
Distribution Agreement In presenting this thesis or dissertation as a partial fulfillment of the requirements for an advanced degree from Emory University, I hereby grant to Emory University and its agents the non-exclusive license to archive, make accessible, and display my thesis or dissertation in whole or in part in all forms of media, now or hereafter known, including display on the world wide web. I understand that I may select some access restrictions as part of the online submission of this thesis or dissertation. I retain all ownership rights to the copyright of the thesis or dissertation. I also retain the right to use in future works (such as articles or books) all or part of this thesis or dissertation. Signature: _____________________________ ______________ Jedidiah Samuel Snyder Date Statistical analysis of concentration-time extrapolation factors for acute inhalation exposures to hazardous substances By Jedidiah S. Snyder Master of Public Health Global Environmental Health _________________________________________ P. Barry Ryan, Ph.D. Committee Chair _________________________________________ Eugene Demchuk, Ph.D. Committee Member _________________________________________ Paige Tolbert, Ph.D. Committee Member Statistical analysis of concentration-time extrapolation factors for acute inhalation exposures to hazardous substances By Jedidiah S. Snyder Bachelor of Science in Engineering, B.S.E. The University of Iowa 2010 Thesis Committee Chair: P. Barry Ryan, Ph.D. An abstract of A thesis submitted to the Faculty of the Rollins School of Public Health of Emory University in partial fulfillment of the requirements for the degree of Master of Public Health in Global Environmental Health 2015 Abstract Statistical analysis of concentration-time extrapolation factors for acute inhalation exposures to hazardous substances By Jedidiah S. -

Verification of Chemical Warfare Agent Exposure in Human Samples
Toxichem Krimtech 2013;80(Special Issue):288 Verification of chemical warfare agent exposure in human samples Paul W. Elsinghorst, Horst Thiermann, Marianne Koller Institut für Pharmakologie und Toxikologie der Bundeswehr, München Abstract Aim: This brief presentation provides an overview of methods that have been developed for the verification of human exposure to chemical warfare agents. Methods: GC–MS detection of nerve agents (V- and G-type) has been carried out with respect to unreacted agents as well as enzyme-bound species and metabolites. Methods involving di- rect SPE from plasma, fluoride-induced release of protein-bound nerve agents in plasma and analysis of their metabolites in plasma and urine have been developed. Exposure to blistering agents, i.e., sulfur mustard, has been verified by GC–MS detection of the unreacted agent in plasma and by LC– and GC–MS analysis of its metabolites in urine. Results: After incorporation nerve agents quickly bind to proteins, e.g., acetylcholinesterase, butyrylcholinesterase or serum albumin, and only small parts remain freely circulating for a few hours (G-type) or up to 2 days (V-type). Concurrently they are converted to O-alkyl methylphosphonic acids by phosphotriesterases and/or simply by aqueous hydrolysis. As a re- sult, different biomarkers can be detected depending on the time passed between exposure and sampling. Unreacted V-type agents can be detected in plasma for 2 days, the O-alkyl methyl- phosphonic acids in plasma for about 2–4 days and in urine for up to 1 week. Fluoride-indu- ced release of protein-bound nerve agents can be carried out until 3 weeks post exposure. -

Toxicological Profile for Glyphosate Were
A f Toxicological Profile for Glyphosate August 2020 GLYPHOSATE II DISCLAIMER Use of trade names is for identification only and does not imply endorsement by the Agency for Toxic Substances and Disease Registry, the Public Health Service, or the U.S. Department of Health and Human Services. GLYPHOSATE III FOREWORD This toxicological profile is prepared in accordance with guidelines developed by the Agency for Toxic Substances and Disease Registry (ATSDR) and the Environmental Protection Agency (EPA). The original guidelines were published in the Federal Register on April 17, 1987. Each profile will be revised and republished as necessary. The ATSDR toxicological profile succinctly characterizes the toxicologic and adverse health effects information for these toxic substances described therein. Each peer-reviewed profile identifies and reviews the key literature that describes a substance's toxicologic properties. Other pertinent literature is also presented, but is described in less detail than the key studies. The profile is not intended to be an exhaustive document; however, more comprehensive sources of specialty information are referenced. The focus of the profiles is on health and toxicologic information; therefore, each toxicological profile begins with a relevance to public health discussion which would allow a public health professional to make a real-time determination of whether the presence of a particular substance in the environment poses a potential threat to human health. The adequacy of information to determine a substance's -

Nerve Agent - Lntellipedia Page 1 Of9 Doc ID : 6637155 (U) Nerve Agent
This document is made available through the declassification efforts and research of John Greenewald, Jr., creator of: The Black Vault The Black Vault is the largest online Freedom of Information Act (FOIA) document clearinghouse in the world. The research efforts here are responsible for the declassification of MILLIONS of pages released by the U.S. Government & Military. Discover the Truth at: http://www.theblackvault.com Nerve Agent - lntellipedia Page 1 of9 Doc ID : 6637155 (U) Nerve Agent UNCLASSIFIED From lntellipedia Nerve Agents (also known as nerve gases, though these chemicals are liquid at room temperature) are a class of phosphorus-containing organic chemicals (organophosphates) that disrupt the mechanism by which nerves transfer messages to organs. The disruption is caused by blocking acetylcholinesterase, an enzyme that normally relaxes the activity of acetylcholine, a neurotransmitter. ...--------- --- -·---- - --- -·-- --- --- Contents • 1 Overview • 2 Biological Effects • 2.1 Mechanism of Action • 2.2 Antidotes • 3 Classes • 3.1 G-Series • 3.2 V-Series • 3.3 Novichok Agents • 3.4 Insecticides • 4 History • 4.1 The Discovery ofNerve Agents • 4.2 The Nazi Mass Production ofTabun • 4.3 Nerve Agents in Nazi Germany • 4.4 The Secret Gets Out • 4.5 Since World War II • 4.6 Ocean Disposal of Chemical Weapons • 5 Popular Culture • 6 References and External Links --------------- ----·-- - Overview As chemical weapons, they are classified as weapons of mass destruction by the United Nations according to UN Resolution 687, and their production and stockpiling was outlawed by the Chemical Weapons Convention of 1993; the Chemical Weapons Convention officially took effect on April 291997. Poisoning by a nerve agent leads to contraction of pupils, profuse salivation, convulsions, involuntary urination and defecation, and eventual death by asphyxiation as control is lost over respiratory muscles. -

1 Abietic Acid R Abrasive Silica for Polishing DR Acenaphthene M (LC
1 abietic acid R abrasive silica for polishing DR acenaphthene M (LC) acenaphthene quinone R acenaphthylene R acetal (see 1,1-diethoxyethane) acetaldehyde M (FC) acetaldehyde-d (CH3CDO) R acetaldehyde dimethyl acetal CH acetaldoxime R acetamide M (LC) acetamidinium chloride R acetamidoacrylic acid 2- NB acetamidobenzaldehyde p- R acetamidobenzenesulfonyl chloride 4- R acetamidodeoxythioglucopyranose triacetate 2- -2- -1- -β-D- 3,4,6- AB acetamidomethylthiazole 2- -4- PB acetanilide M (LC) acetazolamide R acetdimethylamide see dimethylacetamide, N,N- acethydrazide R acetic acid M (solv) acetic anhydride M (FC) acetmethylamide see methylacetamide, N- acetoacetamide R acetoacetanilide R acetoacetic acid, lithium salt R acetobromoglucose -α-D- NB acetohydroxamic acid R acetoin R acetol (hydroxyacetone) R acetonaphthalide (α)R acetone M (solv) acetone ,A.R. M (solv) acetone-d6 RM acetone cyanohydrin R acetonedicarboxylic acid ,dimethyl ester R acetonedicarboxylic acid -1,3- R acetone dimethyl acetal see dimethoxypropane 2,2- acetonitrile M (solv) acetonitrile-d3 RM acetonylacetone see hexanedione 2,5- acetonylbenzylhydroxycoumarin (3-(α- -4- R acetophenone M (LC) acetophenone oxime R acetophenone trimethylsilyl enol ether see phenyltrimethylsilyl... acetoxyacetone (oxopropyl acetate 2-) R acetoxybenzoic acid 4- DS acetoxynaphthoic acid 6- -2- R 2 acetylacetaldehyde dimethylacetal R acetylacetone (pentanedione -2,4-) M (C) acetylbenzonitrile p- R acetylbiphenyl 4- see phenylacetophenone, p- acetyl bromide M (FC) acetylbromothiophene 2- -5- -
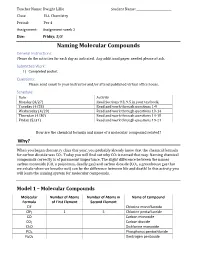
Naming Molecular Compounds General Instructions: Please Do the Activities for Each Day As Indicated
Teacher Name: Dwight Lillie Student Name: ________________________ Class: ELL Chemistry Period: Per 4 Assignment: Assignment week 2 Due: Friday, 5/8 Naming Molecular Compounds General Instructions: Please do the activities for each day as indicated. Any additional paper needed please attach. Submitted Work: 1) Completed packet. Questions: Please send email to your instructor and/or attend published virtual office hours. Schedule: Date Activity Monday (4/27) Read Sections 9.3, 9.5 in your textbook. Tuesday (4/28) Read and work through questions 1-9 Wednesday (4/29) Read and work through questions 10-14 Thursday (4/30) Read and work through questions 14-18 Friday (5/31) Read and work through questions 19-21 How are the chemical formula and name of a molecular compound related? Why? When you began chemistry class this year, you probably already knew that the chemical formula for carbon dioxide was CO2. Today you will find out why CO2 is named that way. Naming chemical compounds correctly is of paramount importance. The slight difference between the names carbon monoxide (CO, a poisonous, deadly gas) and carbon dioxide (CO2, a greenhouse gas that we exhale when we breathe out) can be the difference between life and death! In this activity you will learn the naming system for molecular compounds. Model 1 – Molecular Compounds Molecular Number of Atoms Number of Atoms in Name of Compound Formula of First Element Second Element ClF Chlorine monofluoride ClF5 1 5 Chlorine pentafluoride CO Carbon monoxide CO2 Carbon dioxide Cl2O Dichlorine monoxide PCl5 Phosphorus pentachloride N2O5 Dinitrogen pentoxide 1. Fill in the table to indicate the number of atoms of each type in the molecular formula. -

Nerve Agent Hydrolysis Activity Designed Into a Human Drug Metabolism Enzyme
Nerve Agent Hydrolysis Activity Designed into a Human Drug Metabolism Enzyme Andrew C. Hemmert1, Tamara C. Otto2, Roberto A. Chica3¤, Monika Wierdl4, Jonathan S. Edwards1, Steven L. Lewis1, Carol C. Edwards4, Lyudmila Tsurkan4, C. Linn Cadieux2, Shane A. Kasten2, John R. Cashman5, Stephen L. Mayo3, Philip M. Potter4, Douglas M. Cerasoli2, Matthew R. Redinbo1* 1 Department of Biochemistry/Biophysics and Chemistry, University of North Carolina at Chapel Hill, Chapel Hill, North Carolina, United States of America, 2 United States Army Medical Research Institute for Chemical Defense, Aberdeen Proving Ground, Maryland, United States of America, 3 Department of Biology and Chemistry, California Institute of Technology, Pasadena, California, United States of America, 4 Department of Chemical Biology and Therapeutics, St. Jude Children’s Research Hospital, Memphis, Tennessee, United States of America, 5 Human BioMolecular Research Institute, San Diego, California, United States of America Abstract Organophosphorus (OP) nerve agents are potent suicide inhibitors of the essential neurotransmitter-regulating enzyme acetylcholinesterase. Due to their acute toxicity, there is significant interest in developing effective countermeasures to OP poisoning. Here we impart nerve agent hydrolysis activity into the human drug metabolism enzyme carboxylesterase 1. Using crystal structures of the target enzyme in complex with nerve agent as a guide, a pair of histidine and glutamic acid residues were designed proximal to the enzyme’s native catalytic triad. The resultant variant protein demonstrated significantly increased rates of reactivation following exposure to sarin, soman, and cyclosarin. Importantly, the addition of these residues did not alter the high affinity binding of nerve agents to this protein. Thus, using two amino acid substitutions, a novel enzyme was created that efficiently converted a group of hemisubstrates, compounds that can start but not complete a reaction cycle, into bona fide substrates. -

Organic & Biomolecular Chemistry
Organic & Biomolecular Chemistry Accepted Manuscript This is an Accepted Manuscript, which has been through the Royal Society of Chemistry peer review process and has been accepted for publication. Accepted Manuscripts are published online shortly after acceptance, before technical editing, formatting and proof reading. Using this free service, authors can make their results available to the community, in citable form, before we publish the edited article. We will replace this Accepted Manuscript with the edited and formatted Advance Article as soon as it is available. You can find more information about Accepted Manuscripts in the Information for Authors. Please note that technical editing may introduce minor changes to the text and/or graphics, which may alter content. The journal’s standard Terms & Conditions and the Ethical guidelines still apply. In no event shall the Royal Society of Chemistry be held responsible for any errors or omissions in this Accepted Manuscript or any consequences arising from the use of any information it contains. www.rsc.org/obc Page 1 of 7 Organic & Biomolecular Chemistry Journal Name RSCPublishing ARTICLE Selective chromo-fluorogenic detection of DFP (a Sarin and Soman mimic) and DCNP (a Tabun mimic) Cite this: DOI: 10.1039/x0xx00000x with a unique probe based on a boron dipyrromethene (BODIPY) dye Manuscript Received 00th January 2012, Accepted 00th January 2012 Andrea Barba-Bon,a,b Ana M. Costero,a,b* Salvador Gil,a,b Ramón Martínez- a,c,d a,c,d DOI: 10.1039/x0xx00000x Máñez, * and Félix Sancenón www.rsc.org/ A novel colorimetric probe (P4) for the selective differential detection of DFP (a Sarin and Soman mimic) and DCNP (a Tabun mimic) was prepared.