Epidermal Keratinocyte Sensing and Processing of Environmental
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
The Integumentary System, but Shares Some of Skin’S Properties
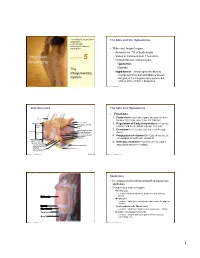
PowerPoint® Lecture Slides The Skin and the Hypodermis prepared by Leslie Hendon University of Alabama, Birmingham • Skin—our largest organ • Accounts for 7% of body weight • Varies in thickness from 1.5–4.4mm C H A P T E R 5 • Divided into two distinct layers • Epidermis The • Dermis Integumentary • Hypodermis—lies deep to the dermis • Composed of areolar and adipose tissues System • Not part of the integumentary system, but shares some of skin’s properties Copyright © 2011 Pearson Education, Inc. Copyright © 2011 Pearson Education, Inc. Skin Structure The Skin and Hypodermis • Functions 1. Protection—cushions organs and protects from Hair shaft bumps, chemicals, water loss, UV radiation Dermal papillae Epidermis Subpapillary vascular 2. Regulation of body temperature---Capillary Papillary plexus network and sweat glands regulate heat loss layer Pore Appendages of skin 3. Excretion—urea, salts, and water lost through Dermis Reticular Eccrine sweat gland sweat layer Arrector pili muscle Sebaceous (oil) gland Hair follicle 4. Production of vitamin D---Epidermal cells use Hair root UV radiation to synthesize vitamin D Hypodermis (superficial fascia) 5. Sensory reception—Contains sense organs Nervous structures Sensory nerve fiber Dermal vascular plexus associated with nerve endings Lamellar (Pacinian) corpuscle Adipose tissue Hair follicle receptor (root hair plexus) Copyright © 2011 Pearson Education, Inc. Figure 5.1 Copyright © 2011 Pearson Education, Inc. Figure 5.2 Gross structure of skin and underlying tissues. Epidermis • Is composed of keratinized stratified squamous epithelium • Contains four main cell types • Keratinocytes • Location—stratum spinosum; produce keratin a fibrous protein Epidermis • Melanocytes • Location—basal layer; manufacture and secrete the pigment melanin Dermis • Tactile epithelial cells (Merkel cells) Hypodermis • Location—basal layer; attached to sensory nerve endings Deep fascia • Dendritic cells (Langerhans cells) • Location—stratum spinosum; part of immune system; Muscle macrophage-like Copyright © 2011 Pearson Education, Inc. -

The Nail Bed, Part I. the Normal Nail Bed Matrix, Stem Cells, Distal Motion and Anatomy
Central Journal of Dermatology and Clinical Research Review Article *Corresponding author Nardo Zaias, Department of Dermatology Mount Sinai Medical Center, Miami Beach, FL. 33140, 4308 The Nail Bed, Part I. The Normal Alton rd. Suite 750, USA, Email: [email protected] Submitted: 25 November 2013 Nail Bed Matrix, Stem Cells, Distal Accepted: 28 December 2013 Published: 31 December 2013 Copyright Motion and Anatomy © 2014 Zaias Nardo Zaias* OPEN ACCESS Department of Dermatology Mount Sinai Medical Center, USA Abstract The nail bed (NB) has its own matrix that originates from distinctive stem cells. The nail bed matrix stem cells (NBMSC) lie immediately distal to the nail plate (NP) matrix cells and are covered by the keratogenous zone of the most distal NPM (LUNULA). The undivided NBMS cells move distally along the NB basement membrane toward the hyponychium; differentiating and keratinizing at various locations, acting as transit amplifying cells and forming a thin layer of NB corneocytes that contact the overlying onychocytes of the NP, homologous to the inner hair root sheath. At the contact point, the NB corneocytes express CarcinoEmbryonic Antigen (CEA), a glycoprotein-modulating adherence which is also found in hair follicles and tumors. Only when both the NP and the NB are normal do they synchronously move distally. The normal NB keratinizes, expressing keratin K-5 and K-17 without keratohyaline granules. However, during trauma or disease states, it reverts to keratinization with orthokeratosis and expresses K-10, as seen in developmental times. Psoriasis is the only exception. Nail Bed epidermis can express hyperplasia and giant cells in some diseases. -

Science of the Nail Apparatus David A.R
1 CHAPTER 1 Science of the Nail Apparatus David A.R. de Berker 1 and Robert Baran 2 1 Bristol Dermatology Centre , Bristol Royal Infi rmary , Bristol , UK 2 Nail Disease Center, Cannes; Gustave Roussy Cancer Institute , Villejuif , France Gross anatomy and terminology, 1 Venous drainage, 19 Physical properties of nails, 35 Embryology, 3 Effects of altered vascular supply, 19 Strength, 35 Morphogenesis, 3 Nail fold vessels, 19 Permeability, 35 Tissue differentiation, 4 Glomus bodies, 20 Radiation penetration, 37 Factors in embryogenesis, 4 Nerve supply, 21 Imaging of the nail apparatus, 37 Regional anatomy, 5 Comparative anatomy and function, 21 Radiology, 37 Histological preparation, 5 The nail and other appendages, 22 Ultrasound, 37 Nail matrix and lunula, 7 Phylogenetic comparisons, 23 Profi lometry, 38 Nail bed and hyponychium, 9 Physiology, 25 Dermoscopy (epiluminescence), 38 Nail folds, 11 Nail production, 25 Photography, 38 Nail plate, 15 Normal nail morphology, 27 Light, 40 Vascular supply, 18 Nail growth, 28 Other techniques, 41 Arterial supply, 18 Nail plate biochemical analysis, 31 Gross anatomy and terminology with the ventral aspect of the proximal nail fold. The intermediate matrix (germinative matrix) is the epithe- Knowledge of nail unit anatomy and terms is important for lial structure starting at the point where the dorsal clinical and scientific work [1]. The nail is an opalescent win- matrix folds back on itself to underlie the proximal nail. dow through to the vascular nail bed. It is held in place by The ventral matrix is synonymous with the nail bed the nail folds, origin at the matrix and attachment to the nail and starts at the border of the lunula, where the inter- bed. -

The Structure of Hair and Follicles of Mice Carrying the Naked ( N) Gene
Genet. Res., Camb. (1982), 39, pp. 139-148 139 Printed in Great Britain The structure of hair and follicles of mice carrying the naked (N) gene BY KATHRYN A. RAPHAEL*, R. E. CHAPMANf, PENELOPE A. FRITHt AND PAMELA R. PENNYCUIK* *CSIRO, Genetics Research Laboratories, P.O. Box 184, North Ryde, N.S.W., 2113, Australia. -fCSIRO, Division of Animal Production, P.O. Box 239, Blacktown, N.S.W., 2148, Australia (Received 8 September 1981) SUMMARY The hairs and follicles from mice carrying the naked (N) gene have been examined using both scanning and transmission electron microscopy in addition to light microscopy. Fibre cuticle cells and occasionally cortical cells were absent from the follicles of N/ + mice when the base of the hair was growing. In N/N follicles there was a frequent lack of both cuticle and cortical cells throughout the growth phase of the follicles. Abnormal- ities were also observed in the manner in which the synthesized keratin was deposited in the fibres. The possible mode of action of the N gene is discussed in the light of these results. 1. INTRODUCTION The hairs of mice carrying the naked (N) gene are affected by varying degrees of fragility. In heterozygous naked (N/ +) mice, the hairs exhibit little abnormality until near the end of each cycle of growth, at which time most hairs break off just above the skin surface, leaving the skin bare until the eruption of the next hair coat. In homozygous naked (N/N) mice, few if any hairs erupt during the first hair cycle, although the follicles are active. -

Epidermis : Composed of a Keratinized Stratified Squamous Epithelium
INTEGUMENTARY The skin (integument , cutis ) and its derivatives constitute the integumentary system . It form the external covering of the body and is the largest organ of the body. The skin consists of two main layers : 1-Epidermis : composed of a keratinized stratified squamous epithelium. 2-Dermis: composed of a dense connective tissue. The epidermal derivatives of the skin include the following organ structures and integumentary products : • Hair and hair follicles • sweat gland • sebaceous gland • nail • mammary glands Structure of thick and thin skin • The thickness of the skin varies over the surface of the body , • thick skin is found in palm of hands and feet while the • thin skin (which contains hair follicles) found in the most of the body for example the skin of scalp . • The skin consists of the following layers :- I- Epidermis The epidermis is composed of stratified squamous epithelium in which four distinct layers can be identified . In the case of thick skin , a fifth layer is observed . Beginning with the deepest layer , these are : . Stratum basale , also called Stratum germinativum . Stratum spinosum . Stratum granulosum . Stratum lucidum (limited to thick skin) . Stratum corneum Stratum basale . • The stratum basale is represented by a single layer of cells that rests on the basal lamina stratum germinativum . • The cells are small and are cuboidal to low columnar in shape . They have less cytoplasm than the cells in the layer above ; consequently , their nuclei are more closely spaced . This , in combination with the basophilic cytoplasm of these cells , imparts a noticeable basophilia to the stratum basale . Stratum spinosum . • Is at least several cells thick. -

The Mammary Bud As a Skin Appendage: Unique and Shared Aspects of Development
J Mammary Gland Biol Neoplasia (2006) 11:187–203 DOI 10.1007/s10911-006-9029-x The Mammary Bud as a Skin Appendage: Unique and Shared Aspects of Development Marja L. Mikkola & Sarah E. Millar Published online: 17 November 2006 # Springer Science + Business Media, Inc. 2006 Abstract Like other skin appendages, the embryonic that can begin to explain the diversity of appendage mammary gland develops via extensive epithelial–mesen- formation, and discuss human genetic diseases that affect chymal interactions. Early stages in embryonic mammary appendage morphogenesis. development strikingly resemble analogous steps in the development of hair follicles and teeth. In each case the Keywords Mammary placode . Mammary bud . first morphological sign of development is a localized Appendage . Hair follicle . Tooth . Ectodermal . thickening in the surface epithelium that subsequently Epidermis . Embryo invaginates to form a mammary, hair follicle or tooth bud. Similar sets of intersecting signaling pathways are involved Abbreviations in patterning the mammary, hair follicle and dental ADULT acro-dermato-ungual-lacrimal-tooth syndrome epithelium, directing placode formation, and controlling APC adenomatous Polyposis Coli bud invagination. Despite these similarities, subsequent AREG Amphiregulin events in the formation of these appendages are diverse. AEC ankyloblepharon-ectodermal dysplasia-clefting The mammary bud extends to form a sprout that begins to syndrome branch upon contact with the mammary fat pad. Hair BCC basal cell carcinoma follicles also extend into the underlying mesenchyme, but BMP bone morphogenetic protein instead of branching, hair follicle epithelium folds around a DKK1 Dickkopf 1 condensation of dermal cells. In contrast, teeth undergo a EDA Ectodysplasin more complex folding morphogenesis. -

The Biology and Science of Hair, Skin and Nails Hair There Are
900 E. Hill Ave Suite 380 Knoxville, TN 37915 www.athomeprep.com 1-800-952-0910 The Biology and Science of Hair, Skin and Nails Hair There are approximately 5 million hair follicles on the body with 100-150 thousand located on the scalp. By 22 weeks gestation, the fetus has all of the hair follicles that it will ever have and no more are added during its lifetime. The hair follicles are more dense as a young child and become less so as our body grows. The primary functions of hair are protection against UV rays and heat loss. Hair cells are called trichocytes and are among the most rapidly dividing cells in the body - doubling every 23-72 hours. The trichocytes are located in the hair follicle, which in turn is located in the dermis of the skin. Each hair follicle has a follicular papilla which is fed by capillaries (small blood vessels). The papilla surrounds the bulb from which the hair grows. This entire complex is located in the dermis of the skin overlying the cranium (also known as the scalp). The follicle has two layers, an outer and an inner. The outer layer continues up to the sebaceous gland and the inner follows the hair shaft and ends below the opening of the sebaceous gland. Each follicle also contains an arrector pili muscle which attaches to the outer sheath just below the sebaceous gland. This causes hair to stand on end when you are frightened or scared or cold. The sebaceous gland produces sebum which acts as a natural conditioner. -

The Study of Hair 3
CHAPTER CHAPTER 3 1 2 The Study of Hair 3 4 5 6 NEUTRON ACTIVATION 7 ANALYSIS OF HAIR In 1958, the body of 16-year-old Gaetane 8 Bouchard was discovered in a gravel pit near her home in Edmundston, New Brunswick, 9 across the Canadian–U.S. border from Maine. Numerous stab wounds were found on her body. Witnesses reported seeing Bouchard 10 with her boyfriend John Vollman prior to her disappearance. Circumstantial evidence also 11 linked Vollman with Bouchard. Paint flakes from the place where the couple had been seen together were found in Vollman’s car. 12 Lipstick that matched the color of Bouchard’s lipstick was found on candy in Vollman’s glove 13 compartment. At Bouchard’s autopsy, several strands of hair were found in her hand. This hair was tested 14 using a process known as neutron activation analysis (NAA). NAA tests for the presence and 15 concentration of various elements in a sample. In this case, NAA showed that the hair in Bouchard’s hand contained a ratio of sulfur to 16 phosphorus that was much closer to Vollman’s hair than her own. At the trial, Vollman con- 17 fessed to the murder in light of the hair analysis results. This was the first time NAA hair analysis was used to convict a criminal. ©Stephen J. Krasemann/Photo Researchers, Inc. Investigators search for clues in a gravel pit similar to the one in which Gaetane Bouchard was buried. 48 31559_03_ch03_p048-075.indd 48 10/2/10 2:29:51 Objectives By the end of this chapter you will be able to 3.1 Identify the various parts of a hair. -

Hair Nigel Collins
Hair Nigel Collins There are between 120 000 and 150 000 hairs on a human head. GCSE key words Specialised cells Protein Oxidation Reduction o t o f p Mitosis o T Hair can be cut and sculpted into the latest fashionable shapes and can hold fast to all the colours of the rainbow. This article describes how hair grows and how its physical structure and chemical make-up are affected by hair products. Hair shaft air is an outgrowth from the skin of mammals. Epidermis Layer of cells Some mammals are covered completely and dividing to Hsome are more or less bare, although even the maintain skin Sebaceous gland and at the base barest will have a few hairs lurking in odd corners. secreting sebum onto of the hair the hair follicle to make How does hair grow? the hair Hair grows as a result of cell division within hair Dermis follicles. These are downgrowths of the outer layer of Erector pili muscle the skin, the epidermis, 4 mm or so into the underlying layer, the dermis (Figure 1). At the base of Outer root sheath each hair follicle there are special cells that divide by Inner root sheath mitosis to make new cells — some form layers around the root of the hair, others form the hair shaft. Matrix of hair root, When new cells are added to the base of the hair Hair bulb where cells divide shaft, the older cells are pushed upwards. The hair to produce the root sheath and the also grows because these cells become longer. -

Accessory Structures of the Skin and Their Functions
Copyright EMAP Publishing 2020 This article is not for distribution except for journal club use Clinical Practice Keywords Skin/Hair/Nails/Sweat glands/Sebaceous glands Systems of life This article has been Skin double-blind peer reviewed In this article... l The four main accessory structures of the skin l Structure and function of hair and nails l The role of sweat and sebaceous glands Skin 2: accessory structures of the skin and their functions Key points Author Sandra Lawton is Queen’s Nurse, nurse consultant and clinical lead Accessory structures dermatology, The Rotherham NHS Foundation Trust. of the skin include the hair, nails, Abstract Understanding the skin requires knowledge of its accessory structures. sweat and These originate embryologically from the epidermis and include hair, nails, sweat sebaceous glands glands and sebaceous glands. All are important in the skin’s key functions, including protection, thermoregulation and its sensory roles. This article, the second in a Hair’s primary two-part series, looks at the structure and function of the main accessory structures functions are of the skin. protection, warmth and sensory Citation Lawton S (2020) Skin 2: accessory structures of the skin and their functions. reception Nursing Times [online]; 116; 1, 44-46. Nails protect the tips of the fingers ccessory structures of the skin l Distribution of sweat-gland products; and toes include the hair, nails, sweat l Psychosocial – hair plays an glands and sebaceous glands. important role in determining self The two main types AThese structures embryologi- image and social perceptions of sweat gland – cally originate from the epidermis and are (Bit.ly/RUAccessoryStructures; eccrine and apocrine often termed “appendages”; they can extend Kolarsick et al, 2011; Graham-Brown and – are responsible down through the dermis into the hypo- Bourke, 2006) . -

The Histological Mechanisms of Hair Loss the Histological Mechanisms of Hair Loss
Provisional chapter Chapter 5 The Histological Mechanisms of Hair Loss The Histological Mechanisms of Hair Loss Vsevolodov Eduard Borisovich Vsevolodov Eduard Borisovich Additional information is available at the end of the chapter Additional information is available at the end of the chapter http://dx.doi.org/10.5772/67275 Abstract The growing hair resists pulling out of the skin in particular site, where the keratiniza- tion of hair cortex and hair cuticle cells as well as the cells of the hair inner root sheath (IS) (being in tight contact) are advanced enough to make them rather strong but lower the level where the hair separates from the hair inner root sheath. The hair which does not grow is kept for some time within the skin by the direct contact of the keratinized hair cortex cells with the cells of the hair outer root sheath. Such contact is absent at the phase of growing hair and even in the case of proliferation inhibition in the follicle bulb causing the lack of hair resistance to pulling it out of the skin several days after inhibition induction. Keywords: hair matrix dysplasia, hair break, hair upward promotion, cell proliferation/ evacuation balance 1. Introduction First of all let us remember most briefly the histological structure of the hair follicle (F) (Figure 1) in the phase of stable hair growth [1–3]. The lowest (innermost) part of the hair F is presented by hair bulb including its cambium zone (“matrix”), which consists of cells dividing all the time while the hair grows. These cells do not seem to differ from each other. -
LAB-Skin-And-Adnexa-2018.Pdf
Skin Introduction It is easy enough to identify a basic tissue in isolation, but it takes further skill to incorporate the knowledge of these separate tissues and distinguish them as such in a compound tissue organ, such as the skin. These basic tissues will be found in some capacity in every tissue you encounter, and the function of this lab is to help you become more familiar in recognizing these specific tissues in organs. Skin is a great example of how the basic tissues combine to create a compound tissue and organ. It is a tissue composed of three distinct layers: epidermis, dermis and hypodermis. Each layer has specific functions, which are derived from their basic tissue components. Your job during this lab is to focus on identifying these basic tissues within these layers of skin and to think about the specific function they impart to the skin. Learning objectives and activities Using the Virtual Slidebox: A Examine the keratinized stratified squamous epithelium of the epidermis, and identify the modified epithelial exocrine glands. B Analyze the organization of collagen fibers and connective tissue cells in the dermis and hypodermis and interpret their function within the skin. C Locate muscle, peripheral nerve and modified nervous tissues in the skin. D Examine hair and hair follicles and determine that they are derived from the epidermis. E Investigate the anatomy of the growing fingernail and appreciate its relationship to skin. F Complete the self-quiz to test your understanding and master your learning. Epidermis: the epithelium of the skin The epidermis is a specialized epithelium: keratinized stratified squamous.