Epidermis : Composed of a Keratinized Stratified Squamous Epithelium
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Anatomy and Physiology of Hair
Chapter 2 Provisional chapter Anatomy and Physiology of Hair Anatomy and Physiology of Hair Bilgen Erdoğan ğ AdditionalBilgen Erdo informationan is available at the end of the chapter Additional information is available at the end of the chapter http://dx.doi.org/10.5772/67269 Abstract Hair is one of the characteristic features of mammals and has various functions such as protection against external factors; producing sebum, apocrine sweat and pheromones; impact on social and sexual interactions; thermoregulation and being a resource for stem cells. Hair is a derivative of the epidermis and consists of two distinct parts: the follicle and the hair shaft. The follicle is the essential unit for the generation of hair. The hair shaft consists of a cortex and cuticle cells, and a medulla for some types of hairs. Hair follicle has a continuous growth and rest sequence named hair cycle. The duration of growth and rest cycles is coordinated by many endocrine, vascular and neural stimuli and depends not only on localization of the hair but also on various factors, like age and nutritional habits. Distinctive anatomy and physiology of hair follicle are presented in this chapter. Extensive knowledge on anatomical and physiological aspects of hair can contribute to understand and heal different hair disorders. Keywords: hair, follicle, anatomy, physiology, shaft 1. Introduction The hair follicle is one of the characteristic features of mammals serves as a unique miniorgan (Figure 1). In humans, hair has various functions such as protection against external factors, sebum, apocrine sweat and pheromones production and thermoregulation. The hair also plays important roles for the individual’s social and sexual interaction [1, 2]. -

Smooth Muscle A-Actin Is a Marker for Hair Follicle Dermis in Vivoand in Vitro
Smooth muscle a-actin is a marker for hair follicle dermis in vivo and in vitro COLIN A. B. JAHODA1*, AMANDA J. REYNOLDS2•*, CHRISTINE CHAPONNIER2, JAMES C. FORESTER3 and GIULIO GABBIANI* 1Department of Biological Sciences, University of Dundee, Dundee DD1 4HN, Scotland ^Department of Pathology, University of Geneva, 1211 Geneva 4, Switzerland ^Department of Surgery, Ninewells Hospital and Medical School, Dundee, Scotland * Present address: Department of Biological Sciences, University of Durham, Durham DH1 3LE, England Summary We have examined the expression of smooth muscle cells contained significant quantities of the a-actin a-actin in hair follicles in situ, and in hair follicle isoform. dermal cells in culture by means of immunohisto- The rapid switching on of smooth muscle a-actin chemistry. Smooth muscle a-actin was present in the expression by dermal papilla cells in early culture, dermal sheath component of rat vibrissa, rat pelage contrasts with the behaviour of smooth muscle cells and human follicles. Dermal papilla cells within all in vitro, and has implications for control of ex- types of follicles did not express the antigen. How- pression of the antigen in normal adult systems. The ever, in culture a large percentage of both hair very high percentage of positively marked cultured dermal papilla and dermal sheath cells were stained papilla and sheath cells also provides a novel marker by this antibody. The same cells were negative when of cells from follicle dermis, and reinforces the idea tested with an antibody to desmin. Overall, explant- that they represent a specialized cell population, derived skin fibroblasts had relatively low numbers contributing to the heterogeneity of fibroblast cell of positively marked cells, but those from skin types in the skin dermis, and possibly acting as a regions of high hair-follicle density displayed more source of myofibroblasts during wound healing. -
The Integumentary System, but Shares Some of Skin’S Properties
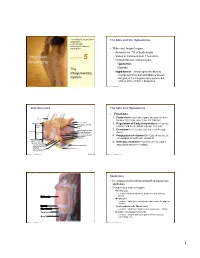
PowerPoint® Lecture Slides The Skin and the Hypodermis prepared by Leslie Hendon University of Alabama, Birmingham • Skin—our largest organ • Accounts for 7% of body weight • Varies in thickness from 1.5–4.4mm C H A P T E R 5 • Divided into two distinct layers • Epidermis The • Dermis Integumentary • Hypodermis—lies deep to the dermis • Composed of areolar and adipose tissues System • Not part of the integumentary system, but shares some of skin’s properties Copyright © 2011 Pearson Education, Inc. Copyright © 2011 Pearson Education, Inc. Skin Structure The Skin and Hypodermis • Functions 1. Protection—cushions organs and protects from Hair shaft bumps, chemicals, water loss, UV radiation Dermal papillae Epidermis Subpapillary vascular 2. Regulation of body temperature---Capillary Papillary plexus network and sweat glands regulate heat loss layer Pore Appendages of skin 3. Excretion—urea, salts, and water lost through Dermis Reticular Eccrine sweat gland sweat layer Arrector pili muscle Sebaceous (oil) gland Hair follicle 4. Production of vitamin D---Epidermal cells use Hair root UV radiation to synthesize vitamin D Hypodermis (superficial fascia) 5. Sensory reception—Contains sense organs Nervous structures Sensory nerve fiber Dermal vascular plexus associated with nerve endings Lamellar (Pacinian) corpuscle Adipose tissue Hair follicle receptor (root hair plexus) Copyright © 2011 Pearson Education, Inc. Figure 5.1 Copyright © 2011 Pearson Education, Inc. Figure 5.2 Gross structure of skin and underlying tissues. Epidermis • Is composed of keratinized stratified squamous epithelium • Contains four main cell types • Keratinocytes • Location—stratum spinosum; produce keratin a fibrous protein Epidermis • Melanocytes • Location—basal layer; manufacture and secrete the pigment melanin Dermis • Tactile epithelial cells (Merkel cells) Hypodermis • Location—basal layer; attached to sensory nerve endings Deep fascia • Dendritic cells (Langerhans cells) • Location—stratum spinosum; part of immune system; Muscle macrophage-like Copyright © 2011 Pearson Education, Inc. -

Culturing of Melanocytes from the Equine Hair Follicle Outer Root Sheath
processes Article Culturing of Melanocytes from the Equine Hair Follicle Outer Root Sheath Hanluo Li 1,† , Jule Kristin Michler 2,† , Alexander Bartella 1 , Anna Katharina Sander 1, Sebastian Gaus 1, Sebastian Hahnel 3, Rüdiger Zimmerer 1, Jan-Christoph Simon 4, Vuk Savkovic 1,*,‡ and Bernd Lethaus 1,‡ 1 Department of Cranial Maxillofacial Plastic Surgery, University Hospital Leipzig, 04103 Leipzig, Germany; [email protected] (H.L.); [email protected] (A.B.); [email protected] (A.K.S.); [email protected] (S.G.); [email protected] (R.Z.); [email protected] (B.L.) 2 Institute of Veterinary Anatomy, University of Leipzig, 04103 Leipzig, Germany; [email protected] 3 Polyclinic for Dental Prosthetics and Material Sciences, University Hospital Leipzig, 04103 Leipzig, Germany; [email protected] 4 Clinic for Dermatology, Venereology and Allergology, University Hospital Leipzig, 04103 Leipzig, Germany; [email protected] * Correspondence: [email protected]; Tel.: +49-341-97-21115 † The first two authors contributed equally to this work. ‡ These authors contributed equally to this work. Abstract: Hair follicles harbor a heterogeneous regenerative cell pool and represent a putative low- to-non-invasively available source of stem cells. We previously reported a technology for culturing human melanocytes from the hair follicle outer root sheath (ORS) for autologous pigmentation of tissue engineered skin equivalents. This study translated the ORS technology to horses. We de-veloped a culture of equine melanocytes from the ORS (eMORS) from equine forelock hair follicles cultured by means of an analogue human hair follicle-based in vitro methodology. -

The Nail Bed, Part I. the Normal Nail Bed Matrix, Stem Cells, Distal Motion and Anatomy
Central Journal of Dermatology and Clinical Research Review Article *Corresponding author Nardo Zaias, Department of Dermatology Mount Sinai Medical Center, Miami Beach, FL. 33140, 4308 The Nail Bed, Part I. The Normal Alton rd. Suite 750, USA, Email: [email protected] Submitted: 25 November 2013 Nail Bed Matrix, Stem Cells, Distal Accepted: 28 December 2013 Published: 31 December 2013 Copyright Motion and Anatomy © 2014 Zaias Nardo Zaias* OPEN ACCESS Department of Dermatology Mount Sinai Medical Center, USA Abstract The nail bed (NB) has its own matrix that originates from distinctive stem cells. The nail bed matrix stem cells (NBMSC) lie immediately distal to the nail plate (NP) matrix cells and are covered by the keratogenous zone of the most distal NPM (LUNULA). The undivided NBMS cells move distally along the NB basement membrane toward the hyponychium; differentiating and keratinizing at various locations, acting as transit amplifying cells and forming a thin layer of NB corneocytes that contact the overlying onychocytes of the NP, homologous to the inner hair root sheath. At the contact point, the NB corneocytes express CarcinoEmbryonic Antigen (CEA), a glycoprotein-modulating adherence which is also found in hair follicles and tumors. Only when both the NP and the NB are normal do they synchronously move distally. The normal NB keratinizes, expressing keratin K-5 and K-17 without keratohyaline granules. However, during trauma or disease states, it reverts to keratinization with orthokeratosis and expresses K-10, as seen in developmental times. Psoriasis is the only exception. Nail Bed epidermis can express hyperplasia and giant cells in some diseases. -

A New Look Into an Old Problem: Keratins As Tools to Investigate Determmanon, Morphogenesis, and Differentiation in Skin
Downloaded from genesdev.cshlp.org on October 10, 2021 - Published by Cold Spring Harbor Laboratory Press A new look into an old problem: keratins as tools to investigate determmanon, morphogenesis, and differentiation in skin Raphael Kopan and Elaine Fuchs Departments of Molecular Genetics and Cell Biology and Biochemistry and Molecular Biology, The University of Chicago, Chicago, Illinois 60637 USA We have investigated keratin and keratin mRNA expression during (1) differentiation of stem cells into epidermis and hair follicles and (2) morphogenesis of follicles. Our results indicate that a type I keratin K14 is expressed early in embryonal basal cells. Subsequently, its expression is elevated in the basal layer of developing epidermis but suppressed in developing matrix cells. This difference represents an early and major biochemical distinction between the two diverging cell types. Moreover, because expression of this keratin is not readily influenced by extracellular regulators or cell culture, it suggests a well-defined and narrow window of development during which an irreversible divergence in basal and matrix cells may take place. In contrast to KI4, which is expressed very early in development and coincident with basal epidermal differentiation, a hair- specific type I keratin and its mRNA is expressed late in hair matrix development and well after follicle morphogenesis. Besides providing an additional developmental difference between epidermal and hair matrix cells, the hair-specific keratins provide the first demonstration that keratin expression may be a consequence rather than a cause of cell organization and differentiation. [Key Words: Hair-specific keratins; keratin mRNA expression; hair follicle morphogenesis] Received September 28, 1988; revised version accepted November 22, 1988. -

Science of the Nail Apparatus David A.R
1 CHAPTER 1 Science of the Nail Apparatus David A.R. de Berker 1 and Robert Baran 2 1 Bristol Dermatology Centre , Bristol Royal Infi rmary , Bristol , UK 2 Nail Disease Center, Cannes; Gustave Roussy Cancer Institute , Villejuif , France Gross anatomy and terminology, 1 Venous drainage, 19 Physical properties of nails, 35 Embryology, 3 Effects of altered vascular supply, 19 Strength, 35 Morphogenesis, 3 Nail fold vessels, 19 Permeability, 35 Tissue differentiation, 4 Glomus bodies, 20 Radiation penetration, 37 Factors in embryogenesis, 4 Nerve supply, 21 Imaging of the nail apparatus, 37 Regional anatomy, 5 Comparative anatomy and function, 21 Radiology, 37 Histological preparation, 5 The nail and other appendages, 22 Ultrasound, 37 Nail matrix and lunula, 7 Phylogenetic comparisons, 23 Profi lometry, 38 Nail bed and hyponychium, 9 Physiology, 25 Dermoscopy (epiluminescence), 38 Nail folds, 11 Nail production, 25 Photography, 38 Nail plate, 15 Normal nail morphology, 27 Light, 40 Vascular supply, 18 Nail growth, 28 Other techniques, 41 Arterial supply, 18 Nail plate biochemical analysis, 31 Gross anatomy and terminology with the ventral aspect of the proximal nail fold. The intermediate matrix (germinative matrix) is the epithe- Knowledge of nail unit anatomy and terms is important for lial structure starting at the point where the dorsal clinical and scientific work [1]. The nail is an opalescent win- matrix folds back on itself to underlie the proximal nail. dow through to the vascular nail bed. It is held in place by The ventral matrix is synonymous with the nail bed the nail folds, origin at the matrix and attachment to the nail and starts at the border of the lunula, where the inter- bed. -

The Structure of Hair and Follicles of Mice Carrying the Naked ( N) Gene
Genet. Res., Camb. (1982), 39, pp. 139-148 139 Printed in Great Britain The structure of hair and follicles of mice carrying the naked (N) gene BY KATHRYN A. RAPHAEL*, R. E. CHAPMANf, PENELOPE A. FRITHt AND PAMELA R. PENNYCUIK* *CSIRO, Genetics Research Laboratories, P.O. Box 184, North Ryde, N.S.W., 2113, Australia. -fCSIRO, Division of Animal Production, P.O. Box 239, Blacktown, N.S.W., 2148, Australia (Received 8 September 1981) SUMMARY The hairs and follicles from mice carrying the naked (N) gene have been examined using both scanning and transmission electron microscopy in addition to light microscopy. Fibre cuticle cells and occasionally cortical cells were absent from the follicles of N/ + mice when the base of the hair was growing. In N/N follicles there was a frequent lack of both cuticle and cortical cells throughout the growth phase of the follicles. Abnormal- ities were also observed in the manner in which the synthesized keratin was deposited in the fibres. The possible mode of action of the N gene is discussed in the light of these results. 1. INTRODUCTION The hairs of mice carrying the naked (N) gene are affected by varying degrees of fragility. In heterozygous naked (N/ +) mice, the hairs exhibit little abnormality until near the end of each cycle of growth, at which time most hairs break off just above the skin surface, leaving the skin bare until the eruption of the next hair coat. In homozygous naked (N/N) mice, few if any hairs erupt during the first hair cycle, although the follicles are active. -

Phagocytosis by Outer Root Sheath Cells on the Mouse Vibrissae
Tu~ ,)oL KNAI or INvunn;A"JJ\ E: DP~MATOLOO\, 62: 54-iii, 1971 Vol. 6:!. Nn. 1 Copyright ® 19i4 b~· The William~ & Wilkir" Co. PrtntPd m U.S.A. PHAGOCYTOSIS BY OUTER ROOT SHEATH CELLS OF THE MOUSE VIBRISSAE (SfNUS HAIRS)* MARlON H . GARRETT. PH.D .. AND KE:-: HASHIMOTO. M. D. ABSTRACT Phagocytic cells are present at all levels of the outer root sheath of mouse vibrissa (sinus hair) follicles. Occasionally these cells are seen in the process of engulfing other cells: more often phagocytic cells are seen which contain one or more ''acuoles whose contents are in various stages of digestion. Most of the phagocytosed cells contain many tonofilaments and a reduced amount of cytoplasm. A few of the vacuoles contam no tilaments. As one vibrissa after another grows from the follicle without an intervening rest period, the outer root sheath changes in size and shape to accommodate both lhe club hair and actively growing vibrissa. Phagocytosis appears to play a role in the continuous reshaping or the outer root sheath Epithelial cells have been shown to be capable of' OBSER\ATIOl'S phagocytosis in response to injury or trauma. Light Microscopy WbjJe some of the injured cells die, other cells in the vicinity have been shown to ingest fibrin [1, 2). The structure of the vibrissa (sinus hair) folLicle serous exudate I 1]. erythrocytes [3 . .q, portions of in mice, in general. corresponds to the description other cells l5]. and injected inert particles [3, 4 j. In given by Melaragno and Montagna [8]. Each cortical cells of the hair matrix, phagocytosis follide is surrounded bv a blood sinus which, in occurs as a normal process as the cells ingest tum, is surrounded by a connective tissue capsule. -

Skin Appendage-Derived Stem Cells: Cell Biology and Potential for Wound Repair Jiangfan Xie1, Bin Yao1,2, Yutong Han3, Sha Huang1,4* and Xiaobing Fu1,4*
Xie et al. Burns & Trauma (2016) 4:38 DOI 10.1186/s41038-016-0064-6 REVIEW Open Access Skin appendage-derived stem cells: cell biology and potential for wound repair Jiangfan Xie1, Bin Yao1,2, Yutong Han3, Sha Huang1,4* and Xiaobing Fu1,4* Abstract Stem cells residing in the epidermis and skin appendages are imperative for skin homeostasis and regeneration. These stem cells also participate in the repair of the epidermis after injuries, inducing restoration of tissue integrity and function of damaged tissue. Unlike epidermis-derived stem cells, comprehensive knowledge about skin appendage-derived stem cells remains limited. In this review, we summarize the current knowledge of skin appendage-derived stem cells, including their fundamental characteristics, their preferentially expressed biomarkers, and their potential contribution involved in wound repair. Finally, we will also discuss current strategies, future applications, and limitations of these stem cells, attempting to provide some perspectives on optimizing the available therapy in cutaneous repair and regeneration. Keywords: Skin appendages, Stem cells, Cell biology, Wound healing Background However, the report of autoallergic repair by skin ap- Skin as a barrier for resisting external invasion is pendage-derived progenitor/stem cells remains limited. distributed to every part of the body, which concludes This review aimed primarily to introduce the skin the epidermis and dermis [1]. Morphologically, the appendage-derived progenitor/stem cells, including their epidermis is the structure in the skin’s outermost layer, characteristics, functions, therapeutic potentials, and and it together with its derivative appendages protects limitations as therapeutic tools for wound healing. In the theorganismfromtheoutside,aswellasregulatesthe following sections, we defined skin appendage-derived body temperature and homeostasis [2]. -

Serial Cultivation of Single Keratinocytes from the Outer Root Sheath of Human Scalp Hair Follicles
Serial Cultivation of Single Keratinocytes from the Outer Root Sheath of Human Scalp Hair Follicles A lain Limat, Ph.D. and Friedrich K. N aser, Ph.D. Cosmital SA, Marl y, Switze rl and (Resea rch Compa ny of We ll a AG, Darmstadt, F. R.G.) A m e tho d fo r the isolatio n of o uter root sheath k eratino h air follicle (yielding approximately 1. 5 X 104 cells) con cytes from pluck ed human hair follicles and for their sub sistentl y gave rise to a confluent 35-mm culture dish (with sequent cultivatio n h as been develop ed. T he selective tryp approximately 1.5 X 106 cells) w ithin abo ut 2 weeks. The sinizatio n of o uter root sheath k eratinocytes provided a o u ter root sh eath k eratinocytes can be serially p assaged for sin gle cell su spension of d efined o rigin within the h air fol up to 3 times and also cryopreserved . J In vest Derm ato! licle. T h e 3T3 feede r layer technique suppo rts su stained 87:485- 488, 1986 growth of these cells in that as little as o ne single pluck ed lucked hair folli cles were fi rst used as a convenient bi the fo llicles were transferred in to a 35-mm Petri dish. The re opsy material fo r the study of geneti c disorders and for maining liquid was sucked off and 10 fo llicles covered with 200 diagnosti c purposes. T hereafter, it beca me evident that ILl 0.1 % trypsin (1: 250) and 0.02% EDTA in phosphate-buffered hair fo llicles provide an interesting model system of saline (PBS) without Ca + + and M g + + (pH 7.2) and incubated epithelial ce ll s for more fundamenta l biomedi ca l re for 10 min at 3rc. -

The Mammary Bud As a Skin Appendage: Unique and Shared Aspects of Development
J Mammary Gland Biol Neoplasia (2006) 11:187–203 DOI 10.1007/s10911-006-9029-x The Mammary Bud as a Skin Appendage: Unique and Shared Aspects of Development Marja L. Mikkola & Sarah E. Millar Published online: 17 November 2006 # Springer Science + Business Media, Inc. 2006 Abstract Like other skin appendages, the embryonic that can begin to explain the diversity of appendage mammary gland develops via extensive epithelial–mesen- formation, and discuss human genetic diseases that affect chymal interactions. Early stages in embryonic mammary appendage morphogenesis. development strikingly resemble analogous steps in the development of hair follicles and teeth. In each case the Keywords Mammary placode . Mammary bud . first morphological sign of development is a localized Appendage . Hair follicle . Tooth . Ectodermal . thickening in the surface epithelium that subsequently Epidermis . Embryo invaginates to form a mammary, hair follicle or tooth bud. Similar sets of intersecting signaling pathways are involved Abbreviations in patterning the mammary, hair follicle and dental ADULT acro-dermato-ungual-lacrimal-tooth syndrome epithelium, directing placode formation, and controlling APC adenomatous Polyposis Coli bud invagination. Despite these similarities, subsequent AREG Amphiregulin events in the formation of these appendages are diverse. AEC ankyloblepharon-ectodermal dysplasia-clefting The mammary bud extends to form a sprout that begins to syndrome branch upon contact with the mammary fat pad. Hair BCC basal cell carcinoma follicles also extend into the underlying mesenchyme, but BMP bone morphogenetic protein instead of branching, hair follicle epithelium folds around a DKK1 Dickkopf 1 condensation of dermal cells. In contrast, teeth undergo a EDA Ectodysplasin more complex folding morphogenesis.