Micronekton and Macrozooplankton of The
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Data Structure
Data structure – Water The aim of this document is to provide a short and clear description of parameters (data items) that are to be reported in the data collection forms of the Global Monitoring Plan (GMP) data collection campaigns 2013–2014. The data itself should be reported by means of MS Excel sheets as suggested in the document UNEP/POPS/COP.6/INF/31, chapter 2.3, p. 22. Aggregated data can also be reported via on-line forms available in the GMP data warehouse (GMP DWH). Structure of the database and associated code lists are based on following documents, recommendations and expert opinions as adopted by the Stockholm Convention COP6 in 2013: · Guidance on the Global Monitoring Plan for Persistent Organic Pollutants UNEP/POPS/COP.6/INF/31 (version January 2013) · Conclusions of the Meeting of the Global Coordination Group and Regional Organization Groups for the Global Monitoring Plan for POPs, held in Geneva, 10–12 October 2012 · Conclusions of the Meeting of the expert group on data handling under the global monitoring plan for persistent organic pollutants, held in Brno, Czech Republic, 13-15 June 2012 The individual reported data component is inserted as: · free text or number (e.g. Site name, Monitoring programme, Value) · a defined item selected from a particular code list (e.g., Country, Chemical – group, Sampling). All code lists (i.e., allowed values for individual parameters) are enclosed in this document, either in a particular section (e.g., Region, Method) or listed separately in the annexes below (Country, Chemical – group, Parameter) for your reference. -

Fishes of the Eastern Ross Sea, Antarctica
Polar Biol (2004) 27: 637–650 DOI 10.1007/s00300-004-0632-2 REVIEW Joseph Donnelly Æ Joseph J. Torres Tracey T. Sutton Æ Christina Simoniello Fishes of the eastern Ross Sea, Antarctica Received: 26 November 2003 / Revised: 16 April 2004 / Accepted: 20 April 2004 / Published online: 16 June 2004 Ó Springer-Verlag 2004 Abstract Antarctic fishes were sampled with 41 midwater in Antarctica is dominated by a few fish families and 6 benthic trawls during the 1999–2000 austral (Bathylagidae, Gonostomatidae, Myctophidae and summer in the eastern Ross Sea. The oceanic pelagic Paralepididae) with faunal diversity decreasing south assemblage (0–1,000 m) contained Electrona antarctica, from the Antarctic Polar Front to the continent (Ever- Gymnoscopelus opisthopterus, Bathylagus antarcticus, son 1984; Kock 1992; Kellermann 1996). South of the Cyclothone kobayashii and Notolepis coatsi. These were Polar Front, the majority of meso- and bathypelagic replaced over the shelf by notothenioids, primarily Ple- fishes have circum-Antarctic distributions (McGinnis uragramma antarcticum. Pelagic biomass was low and 1982; Gon and Heemstra 1990). Taken collectively, the concentrated below 500 m. The demersal assemblage fishes are significant contributors to the pelagic biomass was characteristic of East Antarctica and included seven and are important trophic elements, both as predators species each of Artedidraconidae, Bathydraconidae and and prey (Rowedder 1979; Hopkins and Torres 1989; Channichthyidae, ten species of Nototheniidae, and Lancraft et al. 1989, 1991; Duhamel 1998). Over the three species each of Rajidae and Zoarcidae. Common continental slope and shelf, notothenioids dominate the species were Trematomus eulepidotus (36.5%), T. scotti ichthyofauna (DeWitt 1970). Most members of this (32.0%), Prionodraco evansii (4.9%), T. -

Age and Growth of Brauer's Lanternfish Gymnoscopelus Braueri and Rhombic Lanternfish Krefftichthys Anderssoni (Family Myctophidae) in the Scotia Sea, Southern Ocean
Received: 22 July 2019 Accepted: 14 November 2019 DOI: 10.1111/jfb.14206 REGULAR PAPER FISH Age and growth of Brauer's lanternfish Gymnoscopelus braueri and rhombic lanternfish Krefftichthys anderssoni (Family Myctophidae) in the Scotia Sea, Southern Ocean Ryan A. Saunders1 | Silvia Lourenço2,3 | Rui P. Vieira4 | Martin A. Collins1 | Carlos A. Assis5,6 | Jose C. Xavier7,1 1British Antarctic Survey, Natural Environment Research Council, Cambridge, UK Abstract 2MARE - Marine and Environmental Sciences This study examines age and growth of Brauer's lanternfish Gymnoscopelus braueri and Centre, Polytechnic of Leiria, Peniche, Portugal rhombic lanternfish Krefftichthys anderssoni from the Scotia Sea in the Southern Ocean, 3CIIMAR/CIMAR, Interdisciplinary Centre of Marine and Environmental Research, through the analysis of annual growth increments deposited on sagittal otoliths. Oto- University of Porto, Matosinhos, Portugal lith pairs from 177 G. braueri and 118 K. anderssoni were collected in different seasons 4 Centre for Environment Fisheries and from the region between 2004 and 2009. Otolith-edge analysis suggested a seasonal Aquaculture Sciences, Lowestoft, UK change in opaque and hyaline depositions, indicative of an annual growth pattern, 5MARE-ULisboa – Marine and Environmental Sciences Centre, Faculty of Sciences, although variation within the populations of both species was apparent. Age estimates University of Lisbon, Lisbon, Portugal varied from 1 to 6 years for G. braueri (40 to 139 mm standard length; LS)andfrom 6Department of Animal Biology, Faculty of Sciences, University of Lisbon, Lisbon, Portugal 0 to 2 years for K. anderssoni (26 to 70 mm LS). Length-at-age data were broadly con- 7Department of Life Sciences, MARE-UC, sistent with population cohort parameters identified in concurrent length-frequency University of Coimbra, Coimbra, Portugal data from the region for both species. -

Diet and Trophic Niche of Antarctic Silverfish Pleuragramma
Journal of Fish Biology (2013) 82, 141–164 doi:10.1111/j.1095-8649.2012.03476.x, available online at wileyonlinelibrary.com Diet and trophic niche of Antarctic silverfish Pleuragramma antarcticum in the Ross Sea, Antarctica M. H. Pinkerton*, J. Forman, S. J. Bury, J. Brown, P. Horn and R. L. O’Driscoll National Institute of Water and Atmospheric Research Ltd, Private Bag 14901, Wellington 6241, New Zealand (Received 16 April 2012, Accepted 18 September 2012) The diet of Antarctic silverfish Pleuragramma antarcticum was evaluated by examining stomach ◦ ◦ ◦ ◦ contents of specimens collected in the Ross Sea (71 –77 S; 165 –180 E) in January to March 2008. Pleuragramma antarcticum (50–236 mm standard length, LS) and prey items were analysed for stable-isotopic composition of carbon and nitrogen. According to index of relative importance (IRI), which incorporates frequency of occurrence, mass and number of prey items, the most impor- tant prey items were copepods (81%IRI over all specimens), predominantly Metridia gerlachei and Paraeuchaeta sp., with krill and fishes having low IRI (2·2and5·6%IRI overall). According to mass of prey (M) in stomachs, however, fishes (P. antarcticum and myctophids) and krill domi- nated overall diet (48 and 22%M, respectively), with copepods being a relatively minor constituent of overall diet by mass (9·9%M). Piscivory by P. antarcticum occurred mainly in the extreme south- west of the region and near the continental slope. Krill identified to species level in P. antarcticum stomachs were predominantly Euphausia superba (14·1%M) with some Euphausia crystallopho- rias (4·8%M). -

1 Influence of Sea Ice Anomalies on Antarctic Precipitation Using
https://doi.org/10.5194/tc-2019-69 Preprint. Discussion started: 12 June 2019 c Author(s) 2019. CC BY 4.0 License. Influence of Sea Ice Anomalies on Antarctic Precipitation Using Source Attribution Hailong Wang1*, Jeremy Fyke2,3, Jan Lenaerts4, Jesse Nusbaumer5,6, Hansi Singh1, David Noone7, and Philip Rasch1 (1) Pacific Northwest National Laboratory, Richland, WA 5 (2) Los Alamos National Laboratory, Los Alamos, NM (3) Associated Engineering, Vernon, British Columbia, Canada (4) Department of Atmospheric and Oceanic Sciences, University of Colorado at Boulder, Boulder, CO (5) NASA Goddard Institute for Space Studies, New York, NY (6) Center for Climate Systems Research, Columbia University, New York, NY 10 (7) Oregon State University, Corvallis, OR *Correspondence to: [email protected] 1 https://doi.org/10.5194/tc-2019-69 Preprint. Discussion started: 12 June 2019 c Author(s) 2019. CC BY 4.0 License. Abstract We conduct sensitivity experiments using a climate model that has an explicit water source tagging capability forced by prescribed composites of sea ice concentrations (SIC) and corresponding SSTs to understand the impact of sea ice anomalies on regional evaporation, moisture transport, and source– 5 receptor relationships for precipitation over Antarctica. Surface sensible heat fluxes, evaporation, and column-integrated water vapor are larger over Southern Ocean (SO) areas with lower SIC, but changes in Antarctic precipitation and its source attribution with SICs reflect a strong spatial variability. Among the tagged source regions, the Southern Ocean (south of 50°S) contributes the most (40%) to the Antarctic total precipitation, followed by more northerly ocean basins, most notably the S. -

Antartic Peninsula and Tierra Del Fuego: 100
ANTARCTIC PENINSULA & TIERRA DEL FUEGO BALKEMA – Proceedings and Monographs in Engineering, Water and Earth Sciences Antarctic Peninsula & Tierra del Fuego: 100 years of Swedish-Argentine scientific cooperation at the end of the world Edited by Jorge Rabassa & María Laura Borla Proceedings of “Otto Nordenskjöld’s Antarctic Expedition of 1901–1903 and Swedish Scientists in Patagonia: A Symposium”, held in Buenos Aires, La Plata and Ushuaia, Argentina, March 2–7, 2003. LONDON / LEIDEN / NEW YORK / PHILADELPHIA / SINGAPORE Cover photo information: “The Otto Nordenskjöld’s Expedition to Antarctic Peninsula, 1901–1903. The wintering party in front of the hut on Snow Hill, Antarctica, 30th September 1902. From left to right: Bodman, Jonassen, Nordenskjöld, Ekelöf, Åkerlund and Sobral. Photo: G. Bodman. From the book: Otto Nordenskjöld & John Gunnar Andersson, et al., “Antarctica: or, Two Years amongst the Ice of the South Pole” (London: Hurst & Blackett., 1905)”. Taylor & Francis is an imprint of the Taylor & Francis Group, an informa business This edition published in the Taylor & Francis e-Library, 2007. “To purchase your own copy of this or any of Taylor & Francis or Routledge’s collection of thousands of eBooks please go to www.eBookstore.tandf.co.uk.” © 2007 Taylor & Francis Group plc, London, UK All rights reserved. No part of this publication or the information contained herein may be reproduced, stored in a retrieval system, or transmitted in any form or by any means, electronic, mechanical, by photocopying, recording or otherwise, without written prior permission from the publishers. Although all care is taken to ensure the integrity and quality of this publication and the information herein, no responsibility is assumed by the publishers nor the author for any damage to property or persons as a result of operation or use of this publication and/or the information contained herein. -

Main Drivers of Mercury Levels in Southern Ocean Lantern Fish Myctophidae
Main drivers of mercury levels in Southern Ocean Lantern fish Myctophidae José Seco, José Xavier, Paco Bustamante, Joao Coelho, Ryan Saunders, Nicole Ferreira, Sophie Fielding, Miguel Pardal, Gabriele Stowasser, Thainara Viana, et al. To cite this version: José Seco, José Xavier, Paco Bustamante, Joao Coelho, Ryan Saunders, et al.. Main drivers of mercury levels in Southern Ocean Lantern fish Myctophidae. Environmental Pollution, Elsevier, 2020, 264C (114711), 10.1016/j.envpol.2020.114711. hal-02570100 HAL Id: hal-02570100 https://hal.archives-ouvertes.fr/hal-02570100 Submitted on 11 May 2020 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. Main drivers of mercury levels in Southern Ocean Lantern fish Myctophidae José Seco*1,2, José C. Xavier3,4, Paco Bustamante5, João P. Coelho6, Ryan A. Saunders3, Nicole Ferreira1, Sophie Fielding3, Miguel A. Pardal8, Gabriele Stowasser3, Thainara Viana1, Geraint A. Tarling3, Eduarda Pereira1, Andrew S. Brierley2 1 Department of Chemistry and CESAM/REQUIMTE, University of Aveiro, 3810-193 Aveiro, Portugal 2 Pelagic -

Annals 2/Gilmour
Paper in: Patrick N. Wyse Jackson & Mary E. Spencer Jones (eds) (2011) Annals of Bryozoology 3: aspects of the history of research on bryozoans. International Bryozoology Association, Dublin, pp. viii+225. THE STUDY OF POST-PALEOZOIC BRYOZOANS IN RUSSIA 163 History of the study of Post-Paleozoic bryozoans in Russia (Results and Prospects) L.A. Viskova and A.V. Koromyslova A.A. Borissiak Paleontological Institute, Russian Academy of Sciences, Profsoyuznaya ul. 123, Moscow, 117997 Russia 1. Introduction 2. Triassic 3. Jurassic 4. Lower Cretaceous 5. The Upper Cretaceous-Paleogene 6. Neogene 7. Recent 8. Acknowledgements 1. Introduction Although bryozoans are widespread in the post-Paleozoic deposits of various regions of Russia and the former USSR, they have only been irregularly and insufficiently studied. The history of their studies is analyzed based on the works with more or less full information on the bryozoans that existed during different periods of geological time in the interval Triassic-Recent.1 2. Triassic The first data on the taxonomic composition and distribution of bryozoans in the Triassic of Russia date back only by the 1950s. At first they were restricted to sporadic records of species of the Paleozoic genera Dyscritella and Pseudobatostomella (Order Trepostomida). Bryozoans of the first genus come from the Norian Stage of northeastern Russia. In 1949 they were described by Vasilii Petrovich Nekhoroshev (1893–1977), professor, Doctor in Geology and Mineralogy, leading authority on Paleozoic bryozoans and founder of the St Petersburg school of paleobryozoologists (Figure 1) (Gilmour et al. 2008, Nekhorosheva 2008). The Russian Far Eastern geologists and paleontologists Buriy and Zharnikova (1961) and Lazutkina (1963) recorded species of Pseudobatostomella from the Lower Triassic of Yakutia and Southern Primorye. -

Are Global CO2 Levels Changing?
Activity 2.3: Are Global CO2 Levels Changing? Grades 10 – 12 Materials: Description: Part 1 • “Part per million” demonstration Part 1: Are Global CO2 Levels Changing? During this (Becker Bottle from Flinn, serial activity students learn where CO2 data has been collected, dilutions, etc.) how long it has been collected, and then visualize the • Computers with internet access overall trends in the data. Students then compare different Part 2 • Computers with internet access environments to see if CO2 concentrations are changing all and excel around the world or if changes are only occurring in certain • Printers optional locations. Note: The Flinn Becker Bottle Part 2: Climate Change around the World: Students use the can be purchased at: NASA GISS Surface Temperature Station Data website http://www.flinnsci.com/store/Sc (GISSTEMP http://data.giss.nasa.gov/gistemp/station_data/) ripts/prodView.asp?idproduct=1 to determine whether global CO2 trends students identified 5121 in part 1 are consistent with patterns of temperature change around the world. Students discuss why different locations around the world are affected differently or to different degrees by changing climates. Time: Three class periods for all activities National Science Education Standards A2.c Scientists rely on technology to enhance the gathering and manipulation of data. New techniques and tools provide new evidence to guide inquiry and new methods to gather data, thereby contributing to the advance of science. F3.b Materials from human societies affect both physical and chemical cycles of the Earth. F4.b Human activities can enhance the potential for hazards. AAAS Benchmarks for Science Literacy 8C/M11 By burning fuels, people are releasing large amounts of carbon dioxide into the atmosphere and transforming chemical energy into thermal energy, which spreads throughout the environment. -

100 Million Years of Antarctic Climate Evolution: Evidence from Fossil Plants 19 J
Antarctica: A Keystone in a Changing World Proceedings of the 10th International Symposium on Antarctic Earth Sciences, Santa Barbara, California, August 26 to September 1, 2007, Alan K. Cooper, Peter Barrett, Howard Stagg, Bryan Storey, Edmund Stump, Woody Wise, and the 10th ISAES editorial team, Polar Research Board, National Research Council, U.S. Geological Survey ISBN: 0-309-11855-7, 164 pages, 8 1/2 x 11, (2008) This free PDF was downloaded from: http://www.nap.edu/catalog/12168.html Visit the National Academies Press online, the authoritative source for all books from the National Academy of Sciences, the National Academy of Engineering, the Institute of Medicine, and the National Research Council: • Download hundreds of free books in PDF • Read thousands of books online, free • Sign up to be notified when new books are published • Purchase printed books • Purchase PDFs • Explore with our innovative research tools Thank you for downloading this free PDF. If you have comments, questions or just want more information about the books published by the National Academies Press, you may contact our customer service department toll-free at 888-624-8373, visit us online, or send an email to [email protected]. This free book plus thousands more books are available at http://www.nap.edu. Copyright © National Academy of Sciences. Permission is granted for this material to be shared for noncommercial, educational purposes, provided that this notice appears on the reproduced materials, the Web address of the online, full authoritative version is retained, and copies are not altered. To disseminate otherwise or to republish requires written permission from the National Academies Press. -
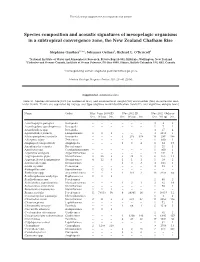
Marine Ecology Progress Series 503:23
The following supplement accompanies the article 1 Species composition and acoustic signatures of mesopelagic organisms in a subtropical convergence zone, the New Zealand Chatham Rise Stéphane Gauthier1, 2,*, Johannes Oeffner1, Richard L. O’Driscoll1 1National Institute of Water and Atmospheric Research, Private Bag 14-901, Kilbirnie, Wellington, New Zealand 2Fisheries and Oceans Canada, Institute of Ocean Sciences, PO Box 6000, Sidney, British Columbia V8L 4B2, Canada *Corresponding author: [email protected] Marine Ecology Progress Series: 503: 23–40 (2014) Supplement. Additional data Table S1. Species occurrence (Occ.) in number of tows, and abundance in weight (Wt) and number (No.) in successful mid - water trawls. Trawls are separated by voyage and type (daytime mark identification trawls ID, and nighttime oblique tows) Name Order May−June 2008 ID Nov 2011 ID Nov 2011 Oblique Occ. Wt (g) No. Occ. Wt (g) No. Occ. Wt (g) No. Acanthephyra pelagica Decapoda − − − − − − 3 9 3 Acanthephyra quadrispinosa Decapoda − − − − − − 1 7 1 Acanthephyra spp. Decapoda − − − − − − 3 17 4 Agrostichthys parkeri Lampriformes 1 8 1 − − − 3 1813 3 Alainopasiphaea australis Decapoda − − − 3 273 356 14 287 366 Allocyttus niger Zeiformes − − − − − − 1 600 1 Amphipod (unspecified) Amphipoda − − − 1 3 4 8 34 17 Anoplogaster cornuta Beryciformes − − − − − − 1 25 1 Apristurus spp. Carcharhiniformes − − − − − − 1 900 1 Argentina elongata Argentiniformes − − − − − − 4 541 6 Argyropelecus gigas Stomiiformes 2 20 2 2 38 5 8 165 12 Argyropelecus hemigymnus Stomiiformes 4 12 6 1 1 1 1 19 2 Astronesthes spp. Stomiiformes − − − 1 3 3 3 103 3 Atolla wyvillei Coronatae − − − − − − 4 83 7 Bathophilus ater Stomiiformes 1 12 1 − − − − − − Bathylagus spp. -

Marine Ecology Progress Series 370:239
Vol. 370: 239–247, 2008 MARINE ECOLOGY PROGRESS SERIES Published October 28 doi: 10.3354/meps07673 Mar Ecol Prog Ser Stable isotopes reveal the trophic position and mesopelagic fish diet of female southern elephant seals breeding on the Kerguelen Islands Y. Cherel1,*, S. Ducatez1, C. Fontaine1, P. Richard2, C. Guinet1 1Centre d’Etudes Biologiques de Chizé, UPR 1934 du CNRS, BP 14, 79360 Villiers-en-Bois, France 2Centre de Recherche sur les Ecosystèmes Littoraux Anthropisés, UMR 6217 du CNRS-IFREMER-ULR, Place du Séminaire, BP 5, 17137 L’Houmeau, France ABSTRACT: Trophic interactions between organisms are the main drivers of ecosystem dynamics, but scant dietary information is available for wide-ranging predators during migration. We investi- gated feeding habits of a key consumer of the Southern Ocean, the southern elephant seal Miroun- gia leonina, by comparing its blood δ13C and δ15N values with those of various marine organisms, including crustaceans, squid, fishes, seabirds and fur seals. At the end of winter, δ13C values (–23.1 to –20.1‰) indicate that female elephant seals forage mainly in the vicinity of the Polar Front and in the Polar Frontal Zone. Trophic levels derived from δ15N values (trophic level = 4.6) show that the southern elephant seal is a top consumer in the pelagic ecosystem that is dominated by colossal squid. The mean δ15N value of seals (10.1 ± 0.3‰) indicates that they are not crustacean eaters, but instead feed on crustacean-eating prey. Surprisingly, most of the previously identified prey species have isotope δ13C and δ15N values that do not fit with those of potential food items.