Digestive Enzyme and Alkaline Phosphatase Activities During the Early Stages of Silurus Soldatovi Development LIU Wei1,2, ZHANG Xiu-Mei1,*, WANG Li-Bo3
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Characterization of Prebiotics and Their Synergistic Activities with Lactobacillus Probiotics for Β-Glucuronidase Reduction
ESEARCH ARTICLE R ScienceAsia 45 (2019): 538–546 doi: 10.2306/scienceasia1513-1874.2019.45.538 Characterization of prebiotics and their synergistic activities with Lactobacillus probiotics for β-glucuronidase reduction a, a b a Achara Chaiongkarn ∗, Jirapa Dathong , Wipaporn Phatvej , Premsuda Saman , Chutima Kuanchaa, Lawan Chatanona, Somporn Moonmungmeea a Biodiversity Research Center, Thailand Institute of Scientific and Technological Research, Pathum Thani 12120 Thailand b Expert Center of Innovative Herbal Products, Thailand Institute of Scientific and Technological Research, Pathum Thani 12120 Thailand ∗Corresponding author, e-mail: [email protected] Received 1 Jun 2018 Accepted 28 Oct 2019 ABSTRACT: The role of synbiotics for enriching health and well-being in addition to suppressing disease is gaining interest. Synergistic activities of four candidate prebiotics as exopolysaccharides (EPSs) derived from Lactobacillus fer- mentum TISTR 2514 (EPS-TISTR 2514), Pediococcus acidilactici TISTR 2612 (EPS-TISTR 2612), manno-oligosaccharides and rice syrup-oligosaccharides were characterized and evaluated for decreasing the risk of colorectal cancer (CRC). Results revealed that one or more candidate prebiotics stimulated the growth of Lactobacillus casei DSM 20011, Lactobacillus plantarum DSM 2648, and Lactobacillus rhamnosus DSM 20021 by at least two orders of magnitude higher than positive control (using FOS as carbon source) within 24 h in vitro. Simulated gastrointestinal (pH 1) and α-amylase (pH 7) resistance were tested. Results showed more than 75% remaining after incubation at 37 °C after 6 h for all treatments except rice syrup. L. plantarum DSM 2648 + manno-oligosaccharides (Tr.1), L. plantarum DSM 2648 + EPS-TISTR 2612 (Tr.2), L. rhamnosus DSM 20021 + rice syrup-oligosaccharides (Tr.3), L. -

Reduction of Pectinesterase Activity in a Commercial Enzyme Preparation
Journal of the Science of Food and Agriculture J Sci Food Agric 85:1613–1621 (2005) DOI: 10.1002/jsfa.2154 Reduction of pectinesterase activity in a commercial enzyme preparation by pulsed electric fields: comparison of inactivation kinetic models Joaquın´ Giner, Pascal Grouberman, Vicente Gimeno and Olga Martın´ ∗ Department of Food Technology, University of Lleida, CeRTA-UTPV, ETSEA, Avda Alcalde Rovira Roure 191, 25198-Lleida, Spain Abstract: The inactivation of pectinesterase (PE) in a commercial enzyme preparation (CEP) under high intensity pulsed electric fields (HIPEF) was studied. After desalting and water dilution of the raw CEP, samples were exposed to exponentially decay waveform pulses for up to 463 µs at electric field intensities ranging from 19 to 38 kV cm−1. Pulses were applied in monopolar mode. Experimental data were fitted to a first-order kinetic model as well as to models based on Fermi, Hulsheger¨ or Weibull equations to describe PE inactivation kinetics. Characteristic parameters for each model were calculated. Relationships between some of the parameters and process variables were obtained. The Weibull model yielded the best accuracy factor. The relationship between residual PE and input of electrical energy density was found to be that of exponential decay. 2005 Society of Chemical Industry Keywords: pulsed electric fields; kinetics; pectinesterase; model; inactivation INTRODUCTION It has become customary to use CEPs in fruit and Pectinesterase (PE; EC 3.1.1.11) is a pectic enzyme vegetable juice technology. Depending -

Isolated Co-Lipase Deficiency in Two Brothers
Gut: first published as 10.1136/gut.23.3.243 on 1 March 1982. Downloaded from Gut, 1982, 23, 243-246 Case reports Isolated co-lipase deficiency in two brothers H HILDEBRAND,* B BORGSTROM, A BEKASSY, C ERLANSON-ALBERTSSON, AND I HELIN From the Department ofPaediatrics and the Department ofPhysiological Chemistry, University ofLund, Sweden SUMMARY Two normally developed Assyrian brothers with isolated pancreatic co-lipase deficiency are described. They presented at the age of 5-6 years with loose stools. They had steatorrhoea, and analysis of exocrine pancreatic enzymes in the small intestine showed co-lipase deficiency, while amylase, chymotrypsin, trypsin, and lipase were normal. Intraduodenal infusion of purified co-lipase improved fat digestion measured by the triolein breath test. Their steatorrhoea diminished on treatment with enteric-coated pancreatic enzymes. The first indication for the existence of a co-factor for activities were assayed titrimetrically`5 using p-tosyl-l- pancreatic lipase was reported in 1963.1 In 1969 a arginine methyl ester (TAME) and N-acetyl-L-tyro- heat-stable co-factor was separated from lipase by gel- sine ethyl ester (ATEE), respectively, as substrates. filtration.2 Pure pancreatic lipase is inhibited by bile Lipase and co-lipase were measured titrimetrically salts in concentrations over their critical micellar con- using tributyrate as substrate.4 Total bile salt concen- centrations.3 The function of the co-factor, called co- trations and the ratio of glycine- to taurine-conjugated lipase, is to restore -

Download Product Insert (PDF)
PRODUCT INFORMATION Lysophospholipase D Polyclonal Antibody Item No. 10005375 Overview and Properties Contents: This vial contains 500 µl of peptide affinity-purified antibody. Synonyms: Autotaxin, ENPP2, lysoPLD Immunogen: Peptide from the C-terminal region of rat LysoPLD Species Reactivity: (+) Human, mouse, and rat; other species not tested Form: Liquid Storage: -20°C (as supplied) Stability: ≥1 year Storage Buffer: TBS, pH 7.4, with 50% glycerol, 0.1%l BSA, and 0.02% sodium azide Host: Rabbit Applications: Immunocytochemistry (ICC), Immunohistochemistry (IHC), and Western blot (WB); the recommended starting dilution for ICC is 1:500, 1:80 for IHC, and 1:200 for WB. Other applications were not tested, therefore optimal working concentration/dilution should be determined empirically. Images 1 · · · · · · · 104 kDa · · · · · · · 60 kDa Lane 1: Human cerebella supernatant (40 µg) Immunohistochemistry analysis of formalin-fixed, paraffin-embedded (FFPE) human cerebellum ǎssue aer heat-induced anǎgen retrieval in pH 6.0 citrate buffer. Aer incubaǎon with Lysophospholipase D Polyclonal Anǎbody (Item No. 10005375) at a 1:80 diluǎon, slides were incubated with bioǎnylated secondary anǎbody, followed by alkaline phosphatase-strepavidin and chromogen (DAB). WARNING CAYMAN CHEMICAL THIS PRODUCT IS FOR RESEARCH ONLY - NOT FOR HUMAN OR VETERINARY DIAGNOSTIC OR THERAPEUTIC USE. 1180 EAST ELLSWORTH RD SAFETY DATA ANN ARBOR, MI 48108 · USA This material should be considered hazardous until further information becomes available. Do not ingest, inhale, get in eyes, on skin, or on clothing. Wash thoroughly after handling. Before use, the user must review the complete Safety Data Sheet, which has been sent via email to your institution. PHONE: [800] 364-9897 WARRANTY AND LIMITATION OF REMEDY [734] 971-3335 Buyer agrees to purchase the material subject to Cayman’s Terms and Conditions. -
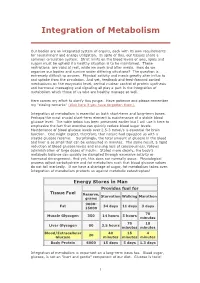
Integration of Metabolism
Integration of Metabolism Our bodies are an integrated system of organs, each with its own requirements for nourishment and energy utilization. In spite of this, our tissues share a common circulation system. Strict limits on the blood levels of ions, lipids and sugars must be upheld if a healthy situation is to be maintained. These restrictions are valid at rest, while we work and after meals. How do we organize our bodies and survive under differing situations? The question is extremely difficult to answer. Physical activity and meals greatly alter influx to and uptake from the circulation. And yet, feedback and feed-forward control mechanisms on the enzymatic level, central nuclear control of protein synthesis and hormonal messaging and signaling all play a part in the integration of metabolism which those of us who are healthy manage so well. Here comes my effort to clarify this jungle. Have patience and please remember my "closing remarks" (click here if you have forgotten them). Integration of metabolism is essential on both short-term and long-term bases. Perhaps the most crucial short-term element is maintenance of a stable blood glucose level. The table below has been presented earlier but I will use it here to emphasize the fact that exercise can quickly reduce blood sugar levels. Maintenance of blood glucose levels over 2.5-3 mmol/s is essential for brain function. One might expect, therefore, that nature had equipped us with a sizable glucose reserve. Surprisingly, the total amount of glucose in the blood and liver is so small that can be exhausted in minutes. -

K113436 B. Purpose for Submi
510(k) SUBSTANTIAL EQUIVALENCE DETERMINATION DECISION SUMMARY ASSAY ONLY TEMPLATE A. 510(k) Number: k113436 B. Purpose for Submission: New device C. Measurand: Alkaline Phosphatase, Amylase, and Lactate Dehydrogenase D. Type of Test: Quantitative, enzymatic activity E. Applicant: Alfa Wassermann Diagnostic Technologies, LLC F. Proprietary and Established Names: ACE Alkaline Phosphatase Reagent Amylase Reagent ACE LDH-L Reagent G. Regulatory Information: Product Classification Regulation Section Panel Code CJE II 862.1050, Alkaline phosphatase 75-Chemistry or isoenzymes test system CIJ II 862.1070, Amylase test system 75-Chemistry CFJ II, exempt, meets 862.1440, Lactate 75-Chemistry limitations of dehydrogenase test system exemption. 21 CFR 862.9 (c) (4) and (9) H. Intended Use: 1. Intended use(s): See indications for use below. 2. Indication(s) for use: The ACE Alkaline Phosphatase Reagent is intended for the quantitative determination of alkaline phosphatase activity in serum using the ACE Axcel Clinical Chemistry System. Measurements of alkaline phosphatase are used in the diagnosis and treatment of liver, bone, parathyroid and intestinal diseases. This test is intended for use in clinical laboratories or physician office laboratories. For in vitro diagnostic use only. The ACE Amylase Reagent is intended for the quantitative determination α-amylase activity in serum using the ACE Axcel Clinical Chemistry System. Amylase measurements are used primarily for the diagnosis and treatment of pancreatitis (inflammation of the pancreas). This test is intended for use in clinical laboratories or physician office laboratories. For in vitro diagnostic use only. The ACE LDH-L Reagent is intended for the quantitative determination of lactate dehydrogenase activity in serum using the ACE Axcel Clinical Chemistry System. -

Extrapancreatic Sources of Lipase
Clinical Chemistry Byline David Parry, PhD, FCACB St. Boniface General Hospital James Dalton, PhD, FCACB Health Sciences Centre Extrapancreatic Sources of Lipase Reports now abound in the literature indicating that serum lipase is more specific and sensitive than amylase for detecting acute pancreatitis in patients presenting with acute abdominal pain (1-7). Amylase has been the mainstay biochemical test for the investigation of acute pancreatitis for many years, whereas lipase has not been widely used primarily because, until recently, reliable lipase assays were not suitable for rapid turnaround of results. Now, reliable and rapid lipase results are available at HSC, St. Boniface General Hospital, Seven Oaks General Hospital and Grace General Hospital. It is generally well appreciated that increases in serum amylase are seen not only in pancreatic disorders, but also in a number of non-pancreatic abdominal and non-abdominal diseases (1). With increased use of lipase, it has become increasingly recognized that lipase too can be elevated in clinical conditions that do not involve the pancreas. Even though the superiority of lipase over amylase for investigating acute pancreatitis has been shown repeatedly in recent literature (2-5), it is important to be aware that there are a number of clinical conditions that can give rise to elevated nonpancreatic lipase: 1) A recent study (2) has shown that some patients presenting with acute abdominal pain have elevations in serum lipase that are due to extrapancreatic disease processes. The sources of elevated lipase were attributed to a number of intra-abdominal anatomic locations other than the pancreas, including the biliary tract, esophagus, stomach, duodenum and small intestine. -

Diagnostic Value of Serum Enzymes-A Review on Laboratory Investigations
Review Article ISSN 2250-0480 VOL 5/ ISSUE 4/OCT 2015 DIAGNOSTIC VALUE OF SERUM ENZYMES-A REVIEW ON LABORATORY INVESTIGATIONS. 1VIDYA SAGAR, M.SC., 2DR. VANDANA BERRY, MD AND DR.ROHIT J. CHAUDHARY, MD 1Vice Principal, Institute of Allied Health Sciences, Christian Medical College, Ludhiana 2Professor & Ex-Head of Microbiology Christian Medical College, Ludhiana 3Assistant Professor Department of Biochemistry Christian Medical College, Ludhiana ABSTRACT Enzymes are produced intracellularly, and released into the plasma and body fluids, where their activities can be measured by their abilities to accelerate the particular chemical reactions they catalyze. But different serum enzymes are raised when different tissues are damaged. So serum enzyme determination can be used both to detect cellular damage and to suggest its location in situ. Some of the biochemical markers such as alanine aminotransferase, aspartate aminotransferase, alkaline phasphatase, gamma glutamyl transferase, nucleotidase, ceruloplasmin, alpha fetoprotein, amylase, lipase, creatine phosphokinase and lactate dehydrogenase are mentioned to evaluate diseases of liver, pancreas, skeletal muscle, bone, etc. Such enzyme test may assist the physician in diagnosis and treatment. KEYWORDS: Liver Function tests, Serum Amylase, Lipase, CPK and LDH. INTRODUCTION mitochondrial AST is seen in extensive tissue necrosis during myocardial infarction and also in chronic Liver diseases like liver tissue degeneration DIAGNOSTIC SERUM ENZYME and necrosis². But lesser amounts are found in Enzymes are very helpful in the diagnosis of brain, pancreas and lung. Although GPT is plentiful cardiac, hepatic, pancreatic, muscular, skeltal and in the liver and occurs only in the small amount in malignant disorders. Serum for all enzyme tests the other tissues. -

GC-MS Metabolic Profile and -Glucosidase-, -Amylase-, Lipase-, and Acetylcholinesterase-Inhibitory Activities of Eight Peach
molecules Article GC-MS Metabolic Profile and α-Glucosidase-, α-Amylase-, Lipase-, and Acetylcholinesterase-Inhibitory Activities of Eight Peach Varieties Dasha Mihaylova 1,* , Ivelina Desseva 2,* , Aneta Popova 3 , Ivayla Dincheva 4 , Radka Vrancheva 2 , Anna Lante 5 and Albert Krastanov 1 1 Department of Biotechnology, Technological Faculty, University of Food Technologies, 4002 Plovdiv, Bulgaria; [email protected] 2 Department of Analytical Chemistry and Physical Chemistry, Technological Faculty, University of Food Technologies, 4002 Plovdiv, Bulgaria; [email protected] 3 Department of Catering and Tourism, Economics Faculty, University of Food Technologies, 4002 Plovdiv, Bulgaria; [email protected] 4 AgroBioInstitute, Agricultural Academy, 8 Dr. Tsankov Blvd., 1164 Sofia, Bulgaria; [email protected] 5 Department of Agronomy, Food, Natural Resources, Animals, and Environment—DAFNAE, Agripolis, University of Padova, 35020 Legnaro, Italy; [email protected] * Correspondence: [email protected] (D.M.); [email protected] (I.D.) Abstract: The inhibition of certain digestive enzymes by target food matrices represents a new approach in the treatment of socially significant diseases. Proving the ability of fruits to inhibit such enzymes can support the inclusion of specific varieties in the daily diets of patients with diabetes, obesity, Alzheimer’s disease, etc., providing them with much more than just valuable Citation: Mihaylova, D.; Desseva, I.; micro- and macromolecules. The current study aimed atidentifying and comparing the GC-MS Popova, A.; Dincheva, I.; Vrancheva, metabolic profiles of eight peach varieties (“Filina”, “Ufo 4, “Gergana”, “Laskava”, “July Lady”, R.; Lante, A.; Krastanov, A. GC-MS “Flat Queen”, “Evmolpiya”, and “Morsiani 90”) grown in Bulgaria (local and introduced) and to Metabolic Profile and α-Glucosidase-, evaluate the inhibitory potential of their extracts towards α-glucosidase, α-amylase, lipase, and α-Amylase-, Lipase-, and acetylcholinesterase. -

The Role of Phospholipase D (Pld) and Grb2 in Chemotaxis
THE ROLE OF PHOSPHOLIPASE D (PLD) AND GRB2 IN CHEMOTAXIS A thesis submitted in partial fulfillment of the requirements for the degree of Master of Science By KATIE J. KNAPEK B.S., Indiana University of Pennyslvania, 2006 2008 Wright State University WRIGHT STATE UNIVERSITY SCHOOL OF GRADUATE STUDIES December 19, 2008 I HEREBY RECOMMEND THAT THE THESIS PREPARED UNDER MY SUPERVISON BY Katie J. Knapek ENTITLED The Role of Phospholipase D (PLD) and Grb2 in Chemotaxis BE ACCEPTED IN PARTIAL FULFILLMENT OF THE REQUIREMENTS FOR THE DEGREE OF Master of Science. _____________________________ Julian Gomez-Cambronero, Ph. D. Thesis Director _____________________________ Barbara Hull, Ph. D. Program Director Committee on Final Examination _______________________ Julian Gomez-Cambronero, Ph. D. _______________________ Nancy Bigley, Ph. D. _______________________ Mill Miller, Ph. D. ________________________ Joseph F. Thomas Jr., Ph. D. Dean, School of Graduate Studies ABSTRACT Knapek, Katie J. M.S., Department of Biological Sciences, Microbiology and Immunology Program, Wright State University, 2008. The Role of Phospholipase D (PLD) and Grb2 in Chemotaxis. Phospholipase D (PLD) is an enzyme that hydrolyzes phosphatidylcholine yielding choline and phosphatidic acid. PLD is activated by mitogens (lead to cell division) and motogens (leading to cell migration). PLD is known to contribute to cellular proliferation and deregulated expression of PLD has been implicated in several human cancers. PLD has been found to play a role in leukocyte chemotaxis and adhesion as studied through the formation of chemokine gradients. We have established a model of cell migration comprising three cell lines: macrophages RAW 264.7 and LR-5 (for innate defense), and fibroblast COS-7 cells (for wound healing). -
What Is Alkaline Phosphatase? What Are the Symptoms of Low ALP?
What is the treatment for HPP? In 2015, asfotase alfa (Strensiq™) was approved for use in the US, the European Union, and Canada for pediatric-onset HPP, and in Japan for HPP with onset at any age. The medication is an injection given multiple times per week underneath the skin (subcutaneous). It is a recombinant (factory-made) form of ALP that has a bone-targeting component. When patients take asfotase alfa, ALP levels measured in the blood are quite high (often in the multiple thousands). Therefore, measuring ALP levels is typically not helpful once a patient is on therapy. What is Alkaline Phosphatase? What are the symptoms of low ALP? For more information, please contact Patients with hypophosphatasia (HPP) have Low ALP can lead to multiple symptoms of HPP, the Soft Bones Foundation. a low blood alkaline phosphatase (ALP) level. including poor bone mineralization and rickets as well (866) 827-9937 – Toll Free as early tooth loss (prior to age 5 years). It is also (973) 453-3093 – Direct Line thought that PPi may build up in muscle tissue 121 Hawkins Place, #267 ALP is an enzyme (a protein that breaks down and may be responsible for the pain and muscle Boonton, New Jersey 07005 chemicals). Most health providers are aware that weakness that some patients with HPP experience. www.SoftBones.org high ALP levels may indicate medical problems, such as liver disease or other bone diseases besides HPP, but many providers may not recognize that low ALP levels can indicate HPP. Low ALP is important What questions should patients ask Written by because the inability to break down chemicals can their doctors about ALP? Jill Simmons, MD lead to elevated levels of these chemicals, which Associate Professor of Pediatrics, Ian Burr Division can cause multiple problems. -

Multiple Roles of Phosphoinositide-Specific Phospholipase C Isozymes
BMB reports Mini Review Multiple roles of phosphoinositide-specific phospholipase C isozymes Pann-Ghill Suh1,*, Jae-Il Park1, Lucia Manzoli2, Lucio Cocco2, Joanna C. Peak3, Matilda Katan3, Kiyoko Fukami4, Tohru Kataoka5, Sanguk Yun1 & Sung Ho Ryu1 1Division of Molecular and Life Sciences, Pohang University of Science and Technology, Pohang, Korea, 2Cellular Signaling Laboratory, Department of Anatomical Sciences, University of Bologna, Via Irnerio, 48 I-40126, Bologna, Italy, 3Cancer Research UK Centre for Cell and Molecular Biology, Chester Beatty Laboratories, The Institute of Cancer Research, Fulham Road, London SW3 6JB, UK, 4Laboratory of Genome and Biosignal, Tokyo University of Pharmacy and Life Science, 1432-1 Horinouchi, Hachioji, 192-0392 Tokyo, Japan, 5Division of Molecular Biology, Department of Biochemistry and Molecular Biology, Kobe University Graduate School of Medicine, Chuo-ku, Kobe, Japan Phosphoinositide-specific phospholipase C is an effector mole- cellular calcium release (1-3; Fig. 1a). cule in the signal transduction process. It generates two sec- The first evidence of PLC activity was suggested by Hokin et ond messengers, inositol-1,4,5-trisphosphate and diacylglycer- al. in 1953 who reported specific hydrolysis of phospholipids ol from phosphatidylinositol 4,5-bisphosphate. Currently, thir- in pigeon’s pancreas slices after cholinergic stimulation (4). teen mammal PLC isozymes have been identified, and they are The authors showed that the enhanced turnover of phosphor- divided into six groups: PLC-β, -γ, -δ, -ε, -ζ and -η. Sequence ylinositol groups of phosphatidylinositol occurred in cells as a analysis studies demonstrated that each isozyme has more response to a variety of stimuli. In 1983, Streb et al.