Henderson, R.W., J.R. Dixon, P. Soina. 1979
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
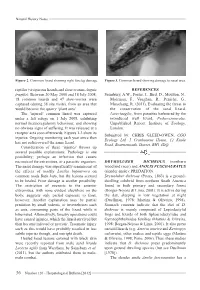
Reptiles (Viviparous Lizards and Slow-Worms Anguis REFERENCES Fragilis)
Natural History Notes Figure 2. Common lizard showing right foreleg damage. Figure 3. Common lizard showing damage to nasal area. reptiles (viviparous lizards and slow-worms Anguis REFERENCES fragilis). Between 20 May 2008 and 18 July 2008, Sainsbury, A.W., Foster, J., Bird, D., Moulton, N., 18 common lizards and 47 slow-worms were Molenaar, F., Vaughan, R., Peniche, G., captured (during 38 site visits), from an area that Marschang, R. (2011). Evaluating the threat to would become the quarry ‘plant area’. the conservation of the sand lizard, The ‘injured’ common lizard was captured Lacertaagilis, from parasites harboured by the under a felt refuge on 1 July 2008, exhibiting introduced wall lizard, Podarcismuralis. normal thermoregulatory behaviour, and showing Unpublished Report. Institute of Zoology, no obvious signs of suffering. It was released at a London. receptor area soon afterwards. Figures 1-3 show its Submitted by: CHRIS GLEED-OWEN, CGO injuries. Ongoing monitoring each year since then Ecology Ltd, 5 Cranbourne House, 12 Knole has not rediscovered the same lizard. Road, Bournemouth, Dorset, BH1 4DQ. Consideration of these ‘injuries’ throws up several possible explanations. Pathology is one possibility; perhaps an infection that causes necrosis of the extremities, or a parasitic organism. DRYMOLUBER DICHROUS (northern The nasal damage was superficially reminiscent of woodland racer) and ANOLIS FUSCOAURATUS the effects of toadfly Lucilia bufonivora on (slender anole): PREDATION. common toads Bufo bufo, but the lesions seemed Drymoluber dichrous (Peters, 1863) is a ground- to be healed. Frost damage is another possibility. dwelling colubrid from northern South America The restriction of necrosis to the anterior found in both primary and secondary forest extremities, with none evident elsewhere on the (Borges-Nojosa & Lima, 2001). -

Herpetological Journal SHORT NOTE
Volume 28 (April 2018), 93-95 SHORT NOTE Herpetological Journal Published by the British Intersexuality in Helicops infrataeniatus Jan, 1865 Herpetological Society (Dipsadidae: Hydropsini) Ruth A. Regnet1, Fernando M. Quintela1, Wolfgang Böhme2 & Daniel Loebmann1 1Universidade Federal do Rio Grande, Instituto de Ciências Biológicas, Laboratório de Vertebrados. Av. Itália km 8, CEP: 96203-900, Vila Carreiros, Rio Grande, Rio Grande do Sul, Brazil 2Zoologisches Forschungsmuseum A. Koenig, Adenauerallee 160, D-53113 Bonn, Germany Herein, we describe the first case of intersexuality in the are viviparous, and interestingly, H. angulatus exhibits Hydropsini tribe. After examination of 720 specimens both reproductive modes (Rossman, 1984; Aguiar & Di- of Helicops infrataeniatus Jan, 1865, we discovered Bernardo, 2005; Braz et al., 2016). Helicops infrataeniatus one individual that presented feminine and masculine has a wide distribution that encompasses south- reproductive features. The specimen was 619 mm long, southeastern Brazil, southern Paraguay, North-eastern with seven follicles in secondary stage, of different shapes Argentina and Uruguay (Deiques & Cechin, 1991; Giraudo, and sizes, and a hemipenis with 13.32 and 13.57 mm in 2001; Carreira & Maneyro, 2013). At the coastal zone of length. The general shape of this organ is similar to that southernmost Brazil, H. infrataeniatus is among the most observed in males, although it is smaller and does not abundant species in many types of limnic and estuarine present conspicuous spines along its body. Deformities environments (Quintela & Loebmann, 2009; Regnet found in feminine and masculine structures suggest that et al., 2017). In October 2015 at the Laranjal beach, this specimen might not be reproductively functional. municipality of Pelotas, state of Rio Grande do Sul, Brazil (31°46’S, 52°13’W), a remarkable aggregation of reptiles Key words: Follicles, hemipenis, hermaphroditism, water and caecilians occurred after a flood event associated to snake. -

Herpetological Information Service No
Type Descriptions and Type Publications OF HoBART M. Smith, 1933 through June 1999 Ernest A. Liner Houma, Louisiana smithsonian herpetological information service no. 127 2000 SMITHSONIAN HERPETOLOGICAL INFORMATION SERVICE The SHIS series publishes and distributes translations, bibliographies, indices, and similar items judged useful to individuals interested in the biology of amphibians and reptiles, but unlikely to be published in the normal technical journals. Single copies are distributed free to interested individuals. Libraries, herpetological associations, and research laboratories are invited to exchange their publications with the Division of Amphibians and Reptiles. We wish to encourage individuals to share their bibliographies, translations, etc. with other herpetologists through the SHIS series. If you have such items please contact George Zug for instructions on preparation and submission. Contributors receive 50 free copies. Please address all requests for copies and inquiries to George Zug, Division of Amphibians and Reptiles, National Museum of Natural History, Smithsonian Institution, Washington DC 20560 USA. Please include a self-addressed mailing label with requests. Introduction Hobart M. Smith is one of herpetology's most prolific autiiors. As of 30 June 1999, he authored or co-authored 1367 publications covering a range of scholarly and popular papers dealing with such diverse subjects as taxonomy, life history, geographical distribution, checklists, nomenclatural problems, bibliographies, herpetological coins, anatomy, comparative anatomy textbooks, pet books, book reviews, abstracts, encyclopedia entries, prefaces and forwords as well as updating volumes being repnnted. The checklists of the herpetofauna of Mexico authored with Dr. Edward H. Taylor are legendary as is the Synopsis of the Herpetofalhva of Mexico coauthored with his late wife, Rozella B. -

Xenosaurus Tzacualtipantecus. the Zacualtipán Knob-Scaled Lizard Is Endemic to the Sierra Madre Oriental of Eastern Mexico
Xenosaurus tzacualtipantecus. The Zacualtipán knob-scaled lizard is endemic to the Sierra Madre Oriental of eastern Mexico. This medium-large lizard (female holotype measures 188 mm in total length) is known only from the vicinity of the type locality in eastern Hidalgo, at an elevation of 1,900 m in pine-oak forest, and a nearby locality at 2,000 m in northern Veracruz (Woolrich- Piña and Smith 2012). Xenosaurus tzacualtipantecus is thought to belong to the northern clade of the genus, which also contains X. newmanorum and X. platyceps (Bhullar 2011). As with its congeners, X. tzacualtipantecus is an inhabitant of crevices in limestone rocks. This species consumes beetles and lepidopteran larvae and gives birth to living young. The habitat of this lizard in the vicinity of the type locality is being deforested, and people in nearby towns have created an open garbage dump in this area. We determined its EVS as 17, in the middle of the high vulnerability category (see text for explanation), and its status by the IUCN and SEMAR- NAT presently are undetermined. This newly described endemic species is one of nine known species in the monogeneric family Xenosauridae, which is endemic to northern Mesoamerica (Mexico from Tamaulipas to Chiapas and into the montane portions of Alta Verapaz, Guatemala). All but one of these nine species is endemic to Mexico. Photo by Christian Berriozabal-Islas. amphibian-reptile-conservation.org 01 June 2013 | Volume 7 | Number 1 | e61 Copyright: © 2013 Wilson et al. This is an open-access article distributed under the terms of the Creative Com- mons Attribution–NonCommercial–NoDerivs 3.0 Unported License, which permits unrestricted use for non-com- Amphibian & Reptile Conservation 7(1): 1–47. -

THE SNAKES of SURINAM, PART XVI: SUBFAMILY XENO- By: A
-THE SNAKES -OF SURINAM, ---PART XVI: SUBFAMILY --XENO- DONTINAE ( GENERA WAGLEROPHIS., XENODON AND XENO PHOLIS). By: A. Abuys, Jukwerderweg 31, 9901 GL Appinge dam, The Netherlands. Contents: The genus WagZerophis - The genus Xeno don - The genus XenophoZis - References. THE GENUS wAGLEROPHIS ROMANO & HOGE, 1972 This genus contains only one species. Prior to 1972 this species was known as Xenodon merremii (Wagler, 1824). This species is found in Surinam. General data for the genus: Head: The head is short and slightly flattened. The strong neck is only marginally narrower than the head. The relatively large eyes have round pupils. Body: Short and stout with smooth scales. The scales have one apical groove. The formation of the dorsal scales is characteristic for this genus. These are arranged so that the upper rows are at an angle to the lower ones (see figure 1) . Tail: Short. Behaviour: Terrestrial and both nocturnal and di urnal. Food: Frogs, toads, lizards and sometimes snakes, insects or small mammals. Habitat: Damp forest floors near swamps or water. Reproduction: Oviparous. Remarks: When threatened or disturbed, the fore part of the body is inflated and the neck is spread in a cobra-like fashion. The head is al· so raised slightly, but not as high and as 181 Fig. 1. Dorsal scale pattern of Waglerophis. From: Peters & Orejas-Miranda, 1970. vertically as a cobra. This is an aggressive snake which will invariably resort to biting if the warning behaviour described above is not heeded. This genus (in common with the genera Xenodon, Heterodon and Lystrophis) has two enlarged teeth attached to the back of the upper jaw. -

De Los Reptiles Del Yasuní
guía dinámica de los reptiles del yasuní omar torres coordinador editorial Lista de especies Número de especies: 113 Amphisbaenia Amphisbaenidae Amphisbaena bassleri, Culebras ciegas Squamata: Serpentes Boidae Boa constrictor, Boas matacaballo Corallus hortulanus, Boas de los jardines Epicrates cenchria, Boas arcoiris Eunectes murinus, Anacondas Colubridae: Dipsadinae Atractus major, Culebras tierreras cafés Atractus collaris, Culebras tierreras de collares Atractus elaps, Falsas corales tierreras Atractus occipitoalbus, Culebras tierreras grises Atractus snethlageae, Culebras tierreras Clelia clelia, Chontas Dipsas catesbyi, Culebras caracoleras de Catesby Dipsas indica, Culebras caracoleras neotropicales Drepanoides anomalus, Culebras hoz Erythrolamprus reginae, Culebras terrestres reales Erythrolamprus typhlus, Culebras terrestres ciegas Erythrolamprus guentheri, Falsas corales de nuca rosa Helicops angulatus, Culebras de agua anguladas Helicops pastazae, Culebras de agua de Pastaza Helicops leopardinus, Culebras de agua leopardo Helicops petersi, Culebras de agua de Peters Hydrops triangularis, Culebras de agua triángulo Hydrops martii, Culebras de agua amazónicas Imantodes lentiferus, Cordoncillos del Amazonas Imantodes cenchoa, Cordoncillos comunes Leptodeira annulata, Serpientes ojos de gato anilladas Oxyrhopus petolarius, Falsas corales amazónicas Oxyrhopus melanogenys, Falsas corales oscuras Oxyrhopus vanidicus, Falsas corales Philodryas argentea, Serpientes liana verdes de banda plateada Philodryas viridissima, Serpientes corredoras -

Check List the Journal Of
12 1 1838 the journal of biodiversity data 6 February 2016 Check List NOTES ON GEOGRAPHIC DISTRIBUTION Check List 12(1): 1838, 6 February 2016 doi: http://dx.doi.org/10.15560/12.1.1838 ISSN 1809-127X © 2016 Check List and Authors Leptophis ahaetulla (Linnaeus, 1758) (Serpentes, Colubridae): first record for the state of Rio Grande do Sul, Brazil Roberto Baptista de Oliveira1*, Christian Beier2, Giancarlo Ribeiro Bilo3, Tiago Gomes dos Santos3 and Gláucia Maria Funk Pontes4 1 Fundação Zoobotânica do Rio Grande do Sul, Museu de Ciências Naturais, Seção de Zoologia de Vertebrados, Rua Dr. Salvador França 1427, CEP 90690-000, Porto Alegre, RS, Brazil 2 Pontifícia Universidade Católica do Rio Grande do Sul, Museu de Ciências e Tecnologia, Programa de Pós-Graduação em Zoologia, Laboratório de Ornitologia, Av. Ipiranga 6681, CEP 90619-900, Porto Alegre, RS, Brazil 3 Universidade Federal do Pampa, Laboratório de Estudos em Biodiversidade Pampiana (LEBIP), Av. Antônio Trilha 1847, CEP 97300- 000, São Gabriel, RS, Brazil 4 Pontifícia Universidade Católica do Rio Grande do Sul, Museu de Ciências e Tecnologia, Setor de Herpetologia, Av. Ipiranga 6681, CEP 90619-900, Porto Alegre, RS, Brazil * Corresponding author: E-mail: [email protected] Abstract: We present the first record of Leptophis the extreme North to the state of Paraná, occupying a ahaetulla for the State of Rio Grande do Sul, Brazil. wide range of biomes, including the Amazon, Pantanal, Between November and December 2014, and February Cerrado, Caatinga and Atlantic Forest (Cunha and 2015, three specimens were found, respectively: one male Nascimento 1978; Vanzolini et al. -

Snakes: Cultural Beliefs and Practices Related to Snakebites in a Brazilian Rural Settlement Dídac S Fita1, Eraldo M Costa Neto2*, Alexandre Schiavetti3
Fita et al. Journal of Ethnobiology and Ethnomedicine 2010, 6:13 http://www.ethnobiomed.com/content/6/1/13 JOURNAL OF ETHNOBIOLOGY AND ETHNOMEDICINE RESEARCH Open Access ’Offensive’ snakes: cultural beliefs and practices related to snakebites in a Brazilian rural settlement Dídac S Fita1, Eraldo M Costa Neto2*, Alexandre Schiavetti3 Abstract This paper records the meaning of the term ‘offense’ and the folk knowledge related to local beliefs and practices of folk medicine that prevent and treat snake bites, as well as the implications for the conservation of snakes in the county of Pedra Branca, Bahia State, Brazil. The data was recorded from September to November 2006 by means of open-ended interviews performed with 74 individuals of both genders, whose ages ranged from 4 to 89 years old. The results show that the local terms biting, stinging and pricking are synonymous and used as equivalent to offending. All these terms mean to attack. A total of 23 types of ‘snakes’ were recorded, based on their local names. Four of them are Viperidae, which were considered the most dangerous to humans, besides causing more aversion and fear in the population. In general, local people have strong negative behavior towards snakes, killing them whenever possible. Until the antivenom was present and available, the locals used only charms, prayers and homemade remedies to treat or protect themselves and others from snake bites. Nowadays, people do not pay attention to these things because, basically, the antivenom is now easily obtained at regional hospitals. It is under- stood that the ethnozoological knowledge, customs and popular practices of the Pedra Branca inhabitants result in a valuable cultural resource which should be considered in every discussion regarding public health, sanitation and practices of traditional medicine, as well as in faunistic studies and conservation strategies for local biological diversity. -

About the Book the Format Acknowledgments
About the Book For more than ten years I have been working on a book on bryophyte ecology and was joined by Heinjo During, who has been very helpful in critiquing multiple versions of the chapters. But as the book progressed, the field of bryophyte ecology progressed faster. No chapter ever seemed to stay finished, hence the decision to publish online. Furthermore, rather than being a textbook, it is evolving into an encyclopedia that would be at least three volumes. Having reached the age when I could retire whenever I wanted to, I no longer needed be so concerned with the publish or perish paradigm. In keeping with the sharing nature of bryologists, and the need to educate the non-bryologists about the nature and role of bryophytes in the ecosystem, it seemed my personal goals could best be accomplished by publishing online. This has several advantages for me. I can choose the format I want, I can include lots of color images, and I can post chapters or parts of chapters as I complete them and update later if I find it important. Throughout the book I have posed questions. I have even attempt to offer hypotheses for many of these. It is my hope that these questions and hypotheses will inspire students of all ages to attempt to answer these. Some are simple and could even be done by elementary school children. Others are suitable for undergraduate projects. And some will take lifelong work or a large team of researchers around the world. Have fun with them! The Format The decision to publish Bryophyte Ecology as an ebook occurred after I had a publisher, and I am sure I have not thought of all the complexities of publishing as I complete things, rather than in the order of the planned organization. -

Ecological Functions of Neotropical Amphibians and Reptiles: a Review
Univ. Sci. 2015, Vol. 20 (2): 229-245 doi: 10.11144/Javeriana.SC20-2.efna Freely available on line REVIEW ARTICLE Ecological functions of neotropical amphibians and reptiles: a review Cortés-Gomez AM1, Ruiz-Agudelo CA2 , Valencia-Aguilar A3, Ladle RJ4 Abstract Amphibians and reptiles (herps) are the most abundant and diverse vertebrate taxa in tropical ecosystems. Nevertheless, little is known about their role in maintaining and regulating ecosystem functions and, by extension, their potential value for supporting ecosystem services. Here, we review research on the ecological functions of Neotropical herps, in different sources (the bibliographic databases, book chapters, etc.). A total of 167 Neotropical herpetology studies published over the last four decades (1970 to 2014) were reviewed, providing information on more than 100 species that contribute to at least five categories of ecological functions: i) nutrient cycling; ii) bioturbation; iii) pollination; iv) seed dispersal, and; v) energy flow through ecosystems. We emphasize the need to expand the knowledge about ecological functions in Neotropical ecosystems and the mechanisms behind these, through the study of functional traits and analysis of ecological processes. Many of these functions provide key ecosystem services, such as biological pest control, seed dispersal and water quality. By knowing and understanding the functions that perform the herps in ecosystems, management plans for cultural landscapes, restoration or recovery projects of landscapes that involve aquatic and terrestrial systems, development of comprehensive plans and detailed conservation of species and ecosystems may be structured in a more appropriate way. Besides information gaps identified in this review, this contribution explores these issues in terms of better understanding of key questions in the study of ecosystem services and biodiversity and, also, of how these services are generated. -

Short Communication Non-Venomous Snakebites in the Western Brazilian
Revista da Sociedade Brasileira de Medicina Tropical Journal of the Brazilian Society of Tropical Medicine Vol.:52:e20190120: 2019 doi: 10.1590/0037-8682-0120-2019 Short Communication Non-venomous snakebites in the Western Brazilian Amazon Ageane Mota da Silva[1],[2], Viviane Kici da Graça Mendes[3],[4], Wuelton Marcelo Monteiro[3],[4] and Paulo Sérgio Bernarde[5] [1]. Instituto Federal do Acre, Campus de Cruzeiro do Sul, Cruzeiro do Sul, AC, Brasil. [2]. Programa de Pós-Graduação Bionorte, Campus Universitário BR 364, Universidade Federal do Acre, Rio Branco, AC, Brasil. [3]. Universidade do Estado do Amazonas, Manaus, AM, Brasil. [4]. Fundação de Medicina Tropical Dr. Heitor Vieira Dourado, Manaus, AM, Brasil. [5]. Laboratório de Herpetologia, Centro Multidisciplinar, Campus Floresta, Universidade Federal do Acre, Cruzeiro do Sul, AC, Brasil. Abstract Introduction: In this study, we examined the clinical manifestations, laboratory evidence, and the circumstances of snakebites caused by non-venomous snakes, which were treated at the Regional Hospital of Juruá in Cruzeiro do Sul. Methods: Data were collected through patient interviews, identification of the species that were taken to the hospital, and the clinical manifestations. Results: Eight confirmed and four probable cases of non-venomous snakebites were recorded. Conclusions: The symptoms produced by the snakes Helicops angulatus and Philodryas viridissima, combined with their coloration can be confused with venomous snakes (Bothrops atrox and Bothrops bilineatus), thus resulting in incorrect bothropic snakebite diagnosis. Keywords: Serpentes. Dipsadidae. Snakes. Ophidism. Envenomation. Snakes from the families Colubridae and Dipsadidae incidence of cases is recorded (56.1 per 100,000 inhabitants)2. Of are traditionally classified as non-poisonous, despite having these, bites by non-venomous snakes are also computed (Boidae, the Duvernoy's gland and the capacity for producing toxic Colubridae, and Dipsadidae) which, depending on the region, secretions, which eventually cause envenomations1. -

Inventario De Flora Y Fauna En El CBIMA (12.6
ii CRÉDITOS Comité Directivo José Vicente Troya Rodríguez Representante Residente del PNUD en Costa Rica Kryssia Brade Representante Residente Adjunta del PNUD en Costa Rica Coordinado por Miriam Miranda Quirós Coordinadora del proyecto Paisajes Productivos-PNUD Consultores que trabajaron en la realización del estudio José Esteban Jiménez, estudio de plantas vasculares Federico Oviedo Brenes, estudio de plantas vasculares Fabián Araya Yannarella, estudio de hongos Víctor J. Acosta-Chaves, estudios de aves, de anfibios y de reptiles Susana Gutiérrez Acuña, estudio de mamíferos Revisado por el comité editorial PNUD Rafaella Sánchez Ingrid Hernández Jose Daniel Estrada Diseño y diagramación Marvin Rojas San José, Costa Rica, 2019 iii RESUMEN EJECUTIVO El conocimiento sobre la diversidad 84 especies esperadas, 21 especies de biológica que habita los ecosistemas anfibios y 84 especies de reptiles. naturales, tanto boscosos como no boscosos, así como en áreas rurales y La diversidad acá presentada, con urbanas, es la línea base fundamental excepción de los hongos, es entre 100 y para establecer un manejo y una gestión 250% mayor respecto al estudio similar adecuadas sobre la protección, efectuado en el 2001 (FUNDENA 2001). conservación y uso sostenible de los La cantidad de especies sensibles es recursos naturales. En el Corredor baja para cada grupo de organismos Biológico Interurbano Maria Aguilar se respecto al total. El CBIMA posee una encontró un total de 765 especies de gran cantidad de especies de plantas plantas vasculares, (74.9% son nativas nativas con alto potencial para restaurar de Costa Rica y crecen naturalmente en espacios físicos degradados y para el CBIMA, un 3.5% son nativas de Costa utilizar como ornamentales.