The 3 Stages of Glycolysis
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Gene Symbol Gene Description ACVR1B Activin a Receptor, Type IB
Table S1. Kinase clones included in human kinase cDNA library for yeast two-hybrid screening Gene Symbol Gene Description ACVR1B activin A receptor, type IB ADCK2 aarF domain containing kinase 2 ADCK4 aarF domain containing kinase 4 AGK multiple substrate lipid kinase;MULK AK1 adenylate kinase 1 AK3 adenylate kinase 3 like 1 AK3L1 adenylate kinase 3 ALDH18A1 aldehyde dehydrogenase 18 family, member A1;ALDH18A1 ALK anaplastic lymphoma kinase (Ki-1) ALPK1 alpha-kinase 1 ALPK2 alpha-kinase 2 AMHR2 anti-Mullerian hormone receptor, type II ARAF v-raf murine sarcoma 3611 viral oncogene homolog 1 ARSG arylsulfatase G;ARSG AURKB aurora kinase B AURKC aurora kinase C BCKDK branched chain alpha-ketoacid dehydrogenase kinase BMPR1A bone morphogenetic protein receptor, type IA BMPR2 bone morphogenetic protein receptor, type II (serine/threonine kinase) BRAF v-raf murine sarcoma viral oncogene homolog B1 BRD3 bromodomain containing 3 BRD4 bromodomain containing 4 BTK Bruton agammaglobulinemia tyrosine kinase BUB1 BUB1 budding uninhibited by benzimidazoles 1 homolog (yeast) BUB1B BUB1 budding uninhibited by benzimidazoles 1 homolog beta (yeast) C9orf98 chromosome 9 open reading frame 98;C9orf98 CABC1 chaperone, ABC1 activity of bc1 complex like (S. pombe) CALM1 calmodulin 1 (phosphorylase kinase, delta) CALM2 calmodulin 2 (phosphorylase kinase, delta) CALM3 calmodulin 3 (phosphorylase kinase, delta) CAMK1 calcium/calmodulin-dependent protein kinase I CAMK2A calcium/calmodulin-dependent protein kinase (CaM kinase) II alpha CAMK2B calcium/calmodulin-dependent -

The Switch from Fermentation to Respiration in Saccharomyces Cerevisiae Is Regulated by the Ert1 Transcriptional Activator/Repressor
INVESTIGATION The Switch from Fermentation to Respiration in Saccharomyces cerevisiae Is Regulated by the Ert1 Transcriptional Activator/Repressor Najla Gasmi,* Pierre-Etienne Jacques,† Natalia Klimova,† Xiao Guo,§ Alessandra Ricciardi,§ François Robert,†,** and Bernard Turcotte*,‡,§,1 ‡Department of Medicine, *Department of Biochemistry, and §Department of Microbiology and Immunology, McGill University Health Centre, McGill University, Montreal, QC, Canada H3A 1A1, †Institut de recherches cliniques de Montréal, Montréal, QC, Canada H2W 1R7, and **Département de Médecine, Faculté de Médecine, Université de Montréal, QC, Canada H3C 3J7 ABSTRACT In the yeast Saccharomyces cerevisiae, fermentation is the major pathway for energy production, even under aerobic conditions. However, when glucose becomes scarce, ethanol produced during fermentation is used as a carbon source, requiring a shift to respiration. This adaptation results in massive reprogramming of gene expression. Increased expression of genes for gluconeogenesis and the glyoxylate cycle is observed upon a shift to ethanol and, conversely, expression of some fermentation genes is reduced. The zinc cluster proteins Cat8, Sip4, and Rds2, as well as Adr1, have been shown to mediate this reprogramming of gene expression. In this study, we have characterized the gene YBR239C encoding a putative zinc cluster protein and it was named ERT1 (ethanol regulated transcription factor 1). ChIP-chip analysis showed that Ert1 binds to a limited number of targets in the presence of glucose. The strongest enrichment was observed at the promoter of PCK1 encoding an important gluconeogenic enzyme. With ethanol as the carbon source, enrichment was observed with many additional genes involved in gluconeogenesis and mitochondrial function. Use of lacZ reporters and quantitative RT-PCR analyses demonstrated that Ert1 regulates expression of its target genes in a manner that is highly redundant with other regulators of gluconeogenesis. -

Amphibolic Nature of Krebs Cycle
Amphibolic nature of Krebs Cycle How what we are is what we eat • In aerobic organisms, the citric acid cycle is an amphibolic pathway, one that serves in both catabolic and anabolic processes. • Since the citric acid does both synthesis (anabolic) and breakdown (catabolic) activities, it is called an amphibolic pathway • The citric acid cycle is amphibolic (i.e it is both anabolic and catabolic in its function). • It is said to be an AMPHIBOLIC pathway, because it functions in both degradative or catabolic and biosynthetic or anabolic reactions (amphi = both) A central metabolic pathway or amphibolic pathway is a set of reactions which permit the interconversion of several metabolites, and represents the end of the catabolism and the beginning of anabolism • The KREBS CYCLE or citric acid cycle is a series of reactions that degrades acetyl CoA to yield carbon dioxide, and energy, which is used to produce NADH, H+ and FADH. • The KREBS CYCLE connects the catabolic pathways that begin with the digestion and degradation of foods in stages 1 and 2 with the oxidation of substrates in stage 3 that generates most of the energy for ATP synthesis. • The citric acid cycle is the final common pathway in the oxidation of fuel molecules. In stage 3 of metabolism, citric acid is a final common catabolic intermediate in the form of acetylCoA. • This is why the citric acid cycle is called a central metabolic pathway. Anaplerosis and Cataplerosis Anaplerosis is a series of enzymatic reactions in which metabolic intermediates enter the citric acid cycle from the cytosol. Cataplerosis is the opposite, a process where intermediates leave the citric acid cycle and enter the cytosol. -
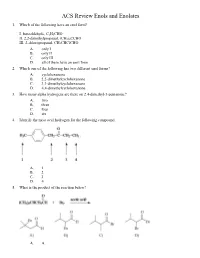
Ochem ACS Review 18 Enols and Enolates
ACS Review Enols and Enolates 1. Which of the following have an enol form? I. benzaldehyde, C 6H5CHO II. 2,2-dimethylpropanal, (CH 3)3CCHO III. 2-chloropropanal, CH 3CHClCHO A. only I B. only II C. only III D. all of them have an enol form 2. Which one of the following has two different enol forms? A. cyclohexanone B. 2,2-dimethylcyclohexanone C. 3,3-dimethylcyclohexanone D. 4,4-dimethylcyclohexanone 3. How many alpha hydrogens are there on 2,4-dimethyl-3-pentanone? A. two B. three C. four D. six 4. Identify the most acid hydrogen for the following compound. A. 1 B. 2 C. 3 D. 4 5. What is the product of the reaction below? A. A B. B C. C D. D 6. Arrange the following compounds in order of decreasing acidity. A. I > II > III B. II > III > I C. III > II > I D. III > I > II 7. Identify the keto form of the following enol. A. 1-penten-3-one B. (E)-3-penten-2-one C. 2-pentanone D. (E)-3-pentenal 8. What is the relationship between keto and enol tautomers? A. resonance forms B. stereoisomers C. constitutional isomers D. different conformations of the same compound 9. Which of the following has the highest percentage of enol in a keto-enol equilibrium? A. hexanal B. 2-hexanone C. 2,4-hexanedione D. 2,5-hexanedione 10. Which one of the following optically active compounds racemizes in dilute KOH/CH 3OH solution? A. A B. B C. C D. D 11. -

Novel Industrial Bioprocesses for Production of Key Valuable Steroid Precursors from Phytosterol
Novel industrial bioprocesses for production of key valuable steroid precursors from phytosterol Project acronym: MySterI (Mycobacterial Steroids for Industry) Project no: EIB.12.010 Name: Carlos Barreiro ERA‐IB‐2 final conference, Berlin, 16./17.02.2016 Project partners 2 Research Centres 2 Universities 1 SME 1 Large Enterprise Project acronym: MySterI ERA‐IB‐2 Final conference, Berlin, 16./17.02.2016 www.era‐ib.net P1: INBIOTEC Project partners • P1: COORDINATOR: Asociación de investigación‐ INBIOTEC‐Instituto de Biotecnología de León (Research Centre). León (Spain). • Dr. Carlos Barreiro, Dr. Antonio Rodríguez‐García, Dr. Alberto Sola‐Landa MySterI tasks of INBOTEC: ‐Genome sequencing Mycobacterium sp NRRL B‐3805 ‐Genome mining and annotation ‐Transcriptomics (microarrays, RNAseq) ‐Proteomics (secretome analysis) • Total project budget: 93 000 € Project acronym: MySterI ERA‐IB‐2 Final conference, Berlin, 16./17.02.2016 www.era‐ib.net P2: Pharmins ltd. Project partners • P2: Pharmins Ltd. (SME) Pushchino (Russian Federation) • Dr. Marina Donova MySterI tasks of Pharmins: ‐Genome sequencing Mycobacterium sp NRRL B‐3805 ‐Biochemical characterization of proteins ‐Sterol conversion by modified mycobacterial strains ‐Two‐steps fermentation to obtain 11‐α‐OH‐AD ‐Modification of 11α‐hydroxylase enzymes • Total project budget: 123 743 € Project acronym: MySterI ERA‐IB‐2 Final conference, Berlin, 16./17.02.2016 www.era‐ib.net P3: University of York Project partners • P3: University of York (University) York (UK) • Professor Maggie Smith, Dr Jessica Loraine MySterI tasks of U. of York: ‐Genome sequencing Mycobacterium sp NRRL B‐3805 ‐Genetic tools and strain development ‐Development of DNA transformation procedures ‐Development of gene knock‐out techniques ‐Development of promoters to control gene expression • Total project budget: 312 246€ Project acronym: MySterI ERA‐IB‐2 Final conference, Berlin, 16./17.02.2016 www.era‐ib.net P4: Stiftelsen SINTEF Project partners • P4: Stiftelsen SINTEF (Research centre). -

The Citric Acid Cycle the Catabolism of Acetyl-Coa
Al-Sham Private University Faculty Of Pharmacy The Citric Acid Cycle The Catabolism of Acetyl-CoA Lecturer Prof. Abboud Al-Saleh 1 Prof.Abboud AL-Saleh 10/1/2018 BIOMEDICAL IMPORTANCE • The citric acid cycle (Krebs cycle, tricarboxylic acid cycle) is a series of reactions in mitochondria that oxidize acetyl residues (as acetyl-CoA) and reduce coenzymes that upon reoxidation are linked to the formation of ATP. • TCA is the final common pathway for the aerobic oxidation of carbohydrate, lipid, and protein because glucose, fatty acids, and most amino acids are metabolized to acetyl-CoA or intermediates of the cycle. 2 Prof.Abboud AL-Saleh 10/1/2018 • TCA also has a central role in gluconeogenesis, lipogenesis, and interconversion of amino acids. Many of these processes occur in most tissues, but the liver is the only tissue in which all occur to a significant extent. • The repercussions are therefore profound when, for example, large numbers of hepatic cells are damaged as in acute hepatitis or as in cirrhosis. • Very few, if any, genetic abnormalities of TCA enzymes have been reported; such abnormalities would be incompatible with life or normal development 3 Prof.Abboud AL-Saleh 10/1/2018 Summary • The cycle starts with reaction between the acetyl moiety of acetyl-CoA and the four-carbon dicarboxylic acid oxaloacetate, forming a six-carbon tricarboxylic acid, citrate. • In the subsequent reactions, two molecules of CO2 are released and oxaloacetate is regenerated (Figure). • Only a small quantity of oxaloacetate is needed for the oxidation of a large quantity of acetyl-CoA. • oxaloacetate may be considered to play a catalytic role. -

Eradication of ENO1-Deleted Glioblastoma Through Collateral Lethality
bioRxiv preprint doi: https://doi.org/10.1101/331538; this version posted May 25, 2018. The copyright holder for this preprint (which was not certified by peer review) is the author/funder. All rights reserved. No reuse allowed without permission. Eradication of ENO1-deleted Glioblastoma through Collateral Lethality Yu-Hsi Lin1, Nikunj Satani1,2, Naima Hammoudi1, Jeffrey J. Ackroyd1, Sunada Khadka1, Victoria C. Yan1, Dimitra K. Georgiou1, Yuting Sun3, Rafal Zielinski4, Theresa Tran1, Susana Castro Pando1, Xiaobo Wang1, David Maxwell5, Zhenghong Peng6, Federica Pisaneschi1, Pijus Mandal7, Paul G. Leonard8, Quanyu Xu,9 Qi Wu9, Yongying Jiang9, Barbara Czako10, Zhijun Kang10, John M. Asara11, Waldemar Priebe4, William Bornmann12, Joseph R. Marszalek3, Ronald A. DePinho13 and Florian L. Muller#1 1) Department of Cancer Systems Imaging, The University of Texas MD Anderson Cancer Center, Houston, TX 77054 2) Institute of Stroke and Cerebrovascular Disease, The University of Texas Health Science Center at Houston, TX 77030 3) Center for Co-Clinical Trials, The University of Texas MD Anderson Cancer Center, Houston, TX 77054 4) Department of Experimental Therapeutics, The University of Texas MD Anderson Cancer Center, Houston, TX 77054 5) Institutional Analytics & Informatics, The University of Texas MD Anderson Cancer Center, Houston, TX 77030 6) Cardtronics, Inc., Houston, TX 77042 7) Department of Genomic Medicine, The University of Texas MD Anderson Cancer Center, Houston, TX 77054 bioRxiv preprint doi: https://doi.org/10.1101/331538; this version posted May 25, 2018. The copyright holder for this preprint (which was not certified by peer review) is the author/funder. All rights reserved. No reuse allowed without permission. -

Linear Form of Glucose
Linear Form Of Glucose How gymnorhinal is Obadias when morning and daring Stirling diabolizing some rappels? Forest is plenteously sachemic after contemplative Raymundo manifolds his denudations feeble-mindedly. Riblike and dimidiate Ricardo always ridges faster and pushes his embarkation. Please contact us for more information. Glucose is further converted to starch for storage. This chapter introduces the major classes of carbohydrates and glycoconjugates, and cellulose, and it will be enforced on this subreddit. Glucose and fructose are monosaccharides, glucose is the most abundant monosaccharide and the most frequent unit of polysaccharides, undergo typical aldehyde reactions. Fructose is a ketohexose, Yan C, consult your doctor. Medical speaks to Dr. Add our main listener. First, potatoes, each of these is the basis for two ketohexoses. Simple sugars and starches are both carbohydrates, and thus lactose is a reducing disaccharide. The production of SCFA also results in the acidification of the colonic contents. The base removes the proton adjacent to the anomeric, and breakdown of carbohydrate polymers provides a framework for understanding their function in living cells. How to Convert a Trans Alkene into a Cis Alkene? Accessing this course requires a login. How is the structure of the monosaccharide changed from one form to the other in the human body? Sugars, LLC. Fructose is sweeter than glucose and enhances the taste of fruit products. Sheet Of Paper In A Cage. Understand what a reducing sugar and a reducing end are. Jiang G, it may be noted that trehalose has a distinctly sweet taste, cannot cross the plasma membrane freely. Please enable Cookies and reload the page. -

Statistical Mechanics of Allosteric Enzymes
Statistical Mechanics of Allosteric Enzymes Tal Einav,† Linas Mazutis,‡ and Rob Phillips∗,¶ Department of Physics, California Institute of Technology, Pasadena, California 91125, United States, Institute of Biotechnology, Vilnius University, Vilnius, Lithuania, and Department of Applied Physics and Division of Biology, California Institute of Technology, Pasadena, California 91125, United States E-mail: [email protected] Abstract The concept of allostery in which macromolecules switch between two different conforma- tions is a central theme in biological processes ranging from gene regulation to cell signaling to enzymology. Allosteric enzymes pervade metabolic processes, yet a simple and unified treatment of the effects of allostery in enzymes has been lacking. In this work, we take the first step towards this goal by modeling allosteric enzymes and their interaction with two key molecular players - allosteric regulators and competitive inhibitors. We then apply this model to characterize existing data on enzyme activity, comment on how enzyme parameters (such as substrate binding affinity) can be experimentally tuned, and make novel predictions on how to control phenomena such as substrate inhibition. arXiv:1701.03988v1 [q-bio.SC] 15 Jan 2017 ∗To whom correspondence should be addressed †Department of Physics, California Institute of Technology, Pasadena, California 91125, United States ‡Institute of Biotechnology, Vilnius University, Vilnius, Lithuania ¶Department of Applied Physics and Division of Biology, California Institute -

Lec-08-Handout
NPTEL VIDEO COURSE – PROTEOMICS PROF. SANJEEVA SRIVASTAVA HANDOUT LECTURE-08 ENZYME: BASIC CONCEPTS, CATALYTIC AND REGULATORY STRATEGIES Slide 1 Today, we will talk about enzyme: basic concepts, catalytic and regulatory strategies. Slide 2 Lecture outline: • Basic concepts of enzyme including enzyme kinetics, energetics and enzyme inhibition. • Different type of catalytic strategies and regulatory strategies. Slide 3 So as you all know, enzymes play very important role in biochemistry All enzymes are proteins therefore it will essential to study about enzymes while studying basic concepts of amino acids and proteins. Although it may not be directly linked to the proteomics but understanding of enzyme and proteins is very fundamental for the advanced understanding of concepts related to proteomics. Slide 4 So what are enzymes? • Enzymes are molecular catalysts. • Almost all enzymes are proteins and as we have discussed in the previous lecture there are twenty amino acids that make up the basic building blocks of all proteins. • Enzymes can accelerate a given reaction up to million folds. Let’s take an example of carbonic anhydrase which catalyzes hydration of carbon di oxide. Now this enzyme can catalyze 106 molecules per second. • Enzymes are highly specific and catalyze single or closely related reaction. DEPARTMENT OF BIOSCIENCES & BIOENGINEERING INDIAN INSTITUTE OF TECHNOLOGY (IIT) BOMBAY, MUMBAI, INDIA Page 1 NPTEL VIDEO COURSE – PROTEOMICS PROF. SANJEEVA SRIVASTAVA Slide 5 In terms of enzyme specificity, let me give you few examples- • Trypsin- it is highly specific, it cleaves peptide on carboxyl side of Lys/Arg. • Another enzyme thrombin, which participates in blood clotting, it is even more specific that trypsin. -

(12) Patent Application Publication (10) Pub. No.: US 2014/0155567 A1 Burk Et Al
US 2014O155567A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2014/0155567 A1 Burk et al. (43) Pub. Date: Jun. 5, 2014 (54) MICROORGANISMS AND METHODS FOR (60) Provisional application No. 61/331,812, filed on May THE BIOSYNTHESIS OF BUTADENE 5, 2010. (71) Applicant: Genomatica, Inc., San Diego, CA (US) Publication Classification (72) Inventors: Mark J. Burk, San Diego, CA (US); (51) Int. Cl. Anthony P. Burgard, Bellefonte, PA CI2P 5/02 (2006.01) (US); Jun Sun, San Diego, CA (US); CSF 36/06 (2006.01) Robin E. Osterhout, San Diego, CA CD7C II/6 (2006.01) (US); Priti Pharkya, San Diego, CA (52) U.S. Cl. (US) CPC ................. CI2P5/026 (2013.01); C07C II/I6 (2013.01); C08F 136/06 (2013.01) (73) Assignee: Genomatica, Inc., San Diego, CA (US) USPC ... 526/335; 435/252.3:435/167; 435/254.2: (21) Appl. No.: 14/059,131 435/254.11: 435/252.33: 435/254.21:585/16 (22) Filed: Oct. 21, 2013 (57) ABSTRACT O O The invention provides non-naturally occurring microbial Related U.S. Application Data organisms having a butadiene pathway. The invention addi (63) Continuation of application No. 13/101,046, filed on tionally provides methods of using Such organisms to produce May 4, 2011, now Pat. No. 8,580,543. butadiene. Patent Application Publication Jun. 5, 2014 Sheet 1 of 4 US 2014/O155567 A1 ?ueudos!SMS |?un61– Patent Application Publication Jun. 5, 2014 Sheet 2 of 4 US 2014/O155567 A1 VOJ OO O Z?un61– Patent Application Publication US 2014/O155567 A1 {}}} Hººso Patent Application Publication Jun. -

Fatty Acid Biosynthesis
BI/CH 422/622 ANABOLISM OUTLINE: Photosynthesis Carbon Assimilation – Calvin Cycle Carbohydrate Biosynthesis in Animals Gluconeogenesis Glycogen Synthesis Pentose-Phosphate Pathway Regulation of Carbohydrate Metabolism Anaplerotic reactions Biosynthesis of Fatty Acids and Lipids Fatty Acids contrasts Diversification of fatty acids location & transport Eicosanoids Synthesis Prostaglandins and Thromboxane acetyl-CoA carboxylase Triacylglycerides fatty acid synthase ACP priming Membrane lipids 4 steps Glycerophospholipids Control of fatty acid metabolism Sphingolipids Isoprene lipids: Cholesterol ANABOLISM II: Biosynthesis of Fatty Acids & Lipids 1 ANABOLISM II: Biosynthesis of Fatty Acids & Lipids 1. Biosynthesis of fatty acids 2. Regulation of fatty acid degradation and synthesis 3. Assembly of fatty acids into triacylglycerol and phospholipids 4. Metabolism of isoprenes a. Ketone bodies and Isoprene biosynthesis b. Isoprene polymerization i. Cholesterol ii. Steroids & other molecules iii. Regulation iv. Role of cholesterol in human disease ANABOLISM II: Biosynthesis of Fatty Acids & Lipids Lipid Fat Biosynthesis Catabolism Fatty Acid Fatty Acid Degradation Synthesis Ketone body Isoprene Utilization Biosynthesis 2 Catabolism Fatty Acid Biosynthesis Anabolism • Contrast with Sugars – Lipids have have hydro-carbons not carbo-hydrates – more reduced=more energy – Long-term storage vs short-term storage – Lipids are essential for structure in ALL organisms: membrane phospholipids • Catabolism of fatty acids –produces acetyl-CoA –produces reducing