Current Threats Faced by Neotropical Parrot Populations MARK ⁎ I
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

TAG Operational Structure
PARROT TAXON ADVISORY GROUP (TAG) Regional Collection Plan 5th Edition 2020-2025 Sustainability of Parrot Populations in AZA Facilities ...................................................................... 1 Mission/Objectives/Strategies......................................................................................................... 2 TAG Operational Structure .............................................................................................................. 3 Steering Committee .................................................................................................................... 3 TAG Advisors ............................................................................................................................... 4 SSP Coordinators ......................................................................................................................... 5 Hot Topics: TAG Recommendations ................................................................................................ 8 Parrots as Ambassador Animals .................................................................................................. 9 Interactive Aviaries Housing Psittaciformes .............................................................................. 10 Private Aviculture ...................................................................................................................... 13 Communication ........................................................................................................................ -

Dominican Republic Endemics of Hispaniola II 1St February to 9Th February 2021 (9 Days)
Dominican Republic Endemics of Hispaniola II 1st February to 9th February 2021 (9 days) Palmchat by Adam Riley Although the Dominican Republic is perhaps best known for its luxurious beaches, outstanding food and vibrant culture, this island has much to offer both the avid birder and general naturalist alike. Because of the amazing biodiversity sustained on the island, Hispaniola ranks highest in the world as a priority for bird protection! This 8-day birding tour provides the perfect opportunity to encounter nearly all of the island’s 32 endemic bird species, plus other Greater Antillean specialities. We accomplish this by thoroughly exploring the island’s variety of habitats, from the evergreen and Pine forests of the Sierra de Bahoruco to the dry forests of the coast. Furthermore, our accommodation ranges from remote cabins deep in the forest to well-appointed hotels on the beach, each with its own unique local flair. Join us for this delightful tour to the most diverse island in the Caribbean! RBL Dominican Republic Itinerary 2 THE TOUR AT A GLANCE… THE ITINERARY Day 1 Arrival in Santo Domingo Day 2 Santo Domingo Botanical Gardens to Sabana del Mar (Paraiso Caño Hondo) Day 3 Paraiso Caño Hondo to Santo Domingo Day 4 Salinas de Bani to Pedernales Day 5 Cabo Rojo & Southern Sierra de Bahoruco Day 6 Cachote to Villa Barrancoli Day 7 Northern Sierra de Bahoruco Day 8 La Placa, Laguna Rincon to Santo Domingo Day 9 International Departures TOUR ROUTE MAP… RBL Dominican Republic Itinerary 3 THE TOUR IN DETAIL… Day 1: Arrival in Santo Domingo. -

Population Size, Provisioning Frequency, Flock Size and Foraging
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Naturalis Population size, provisioning frequency, flock size and foraging range at the largest known colony of Psittaciformes: the Burrowing Parrots of the north-eastern Patagonian coastal cliffs Juan F. MaselloA,B,C,G, María Luján PagnossinD, Christina SommerE and Petra QuillfeldtF AInstitut für Ökologie, Friedrich-Schiller-Universität Jena, Germany. BEcology of Vision Group, School of Biological Sciences, University of Bristol, UK. CMax Planck Institute for Ornithology, Vogelwarte Radolfzell, Schlossallee 2, D-78315 Radolfzell, Germany. DFacultad de Ciencias Naturales y Museo, Universidad Nacional de La Plata, La Plata, Argentina. EInstitut für Biologie/Verhaltensbiologie, Freie Universität Berlin, Berlin, Germany. FSchool of Biosciences, Cardiff University, UK. GCorresponding author. Email: [email protected] Abstract.We here describe the largest colony of Burrowing Parrots (Cyanoliseus patagonus), located in Patagonia, Argentina. Counts during the 2001–02 breeding season showed that the colony extended along 9 km of a sandstone cliff facing the Altantic Ocean, in the province of Río Negro, Patagonia, Argentina, and contained 51412 burrows, an estimated 37527 of which were active. To our knowledge, this is largest known colony of Psittaciformes. Additionally, 6500 Parrots not attending nestlings were found to be associated with the colony during the 2003–04 breeding season. We monitored activities at nests and movements between nesting and feeding areas. Nestlings were fed 3–6 times daily. Adults travelled in flocks of up to 263 Parrots to the feeding grounds in early mornings; later in the day, they flew in smaller flocks, making 1–4 trips to the feeding grounds. -

BIRDCONSERVATION the Magazine of American Bird Conservancy Fall 2016 BIRD’S EYE VIEW a Life Shaped by Migration
BIRDCONSERVATION The Magazine of American Bird Conservancy Fall 2016 BIRD’S EYE VIEW A Life Shaped By Migration The years have rolled by, leaving me with many memories touched by migrating birds. Migrations tell the chronicle of my life, made more poignant by their steady lessening through the years. still remember my first glimmer haunting calls of the cranes and Will the historic development of of understanding of the bird swans together, just out of sight. improved relations between the Imigration phenomenon. I was U.S. and Cuba nonetheless result in nine or ten years old and had The years have rolled by, leaving me the loss of habitats so important to spotted a male Yellow Warbler in with many memories touched by species such as the Black-throated spring plumage. Although I had migrating birds. Tracking a Golden Blue Warbler (page 18)? And will passing familiarity with the year- Eagle with a radio on its back Congress strengthen or weaken the round and wintertime birds at through downtown Milwaukee. Migratory Bird Treaty Act (page home, this springtime beauty was Walking down the Cape May beach 27), America’s most important law new to me. I went to my father for each afternoon to watch the Least protecting migratory birds? an explanation of how I had missed Tern colony. The thrill of seeing this bird before. Dad explained bird “our” migrants leave Colombia to We must address each of these migration, a talk that lit a small pour back north. And, on a recent concerns and a thousand more, fire in me that has never been summer evening, standing outside but we cannot be daunted by their extinguished. -

Factors Influencing Density of the Northern Mealy Amazon in Three Forest Types of a Modified Rainforest Landscape in Mesoamerica
VOLUME 12, ISSUE 1, ARTICLE 5 De Labra-Hernández, M. Á., and K. Renton. 2017. Factors influencing density of the Northern Mealy Amazon in three forest types of a modified rainforest landscape in Mesoamerica. Avian Conservation and Ecology 12(1):5. https://doi.org/10.5751/ACE-00957-120105 Copyright © 2017 by the author(s). Published here under license by the Resilience Alliance. Research Paper Factors influencing density of the Northern Mealy Amazon in three forest types of a modified rainforest landscape in Mesoamerica Miguel Ángel De Labra-Hernández 1 and Katherine Renton 2 1Posgrado en Ciencias Biológicas, Instituto de Biología, Universidad Nacional Autónoma de México, Mexico City, México, 2Estación de Biología Chamela, Instituto de Biología, Universidad Nacional Autónoma de México, Jalisco, México ABSTRACT. The high rate of conversion of tropical moist forest to secondary forest makes it imperative to evaluate forest metric relationships of species dependent on primary, old-growth forest. The threatened Northern Mealy Amazon (Amazona guatemalae) is the largest mainland parrot, and occurs in tropical moist forests of Mesoamerica that are increasingly being converted to secondary forest. However, the consequences of forest conversion for this recently taxonomically separated parrot species are poorly understood. We measured forest metrics of primary evergreen, riparian, and secondary tropical moist forest in Los Chimalapas, Mexico. We also used point counts to estimate density of Northern Mealy Amazons in each forest type during the nonbreeding (Sept 2013) and breeding (March 2014) seasons. We then examined how parrot density was influenced by forest structure and composition, and how parrots used forest types within tropical moist forest. -
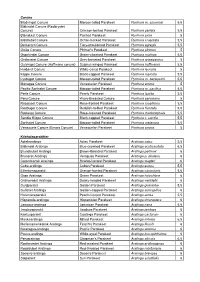
Rødbrystet Conure
Conure Blodvinget Conure Maroon -tailed Parakeet Pyrrhura m. souancei 5,5 Blåkindet Conure (Rødbrystet Conure) Crimson-bellied Parakeet Pyrrhura perlata 5,5 Blånakket Conure Painted Parakeet Pyrrhura picta 5 Blåstrubet Conure Ochre-marked Parakeet Pyrrhura cruentata 5,5 Demerara Conure Fiery-shouldered Parakeet Pyrrhura egregia 5,5 Goiás Conure Pfrimer's Parakeet Pyrrhura pfrimeri 5 Grønkindet Conure Green-cheeked Parakeet Pyrrhura molinae 5,5 Gråbrystet Conure Grey-breasted Parakeet Pyrrhura griseipectus 5 Gulvinget Conure (Hoffmans conure) Sulphur-winged Parakeet Pyrrhura hoffmanni 5,5 Hvidøret Conure White-eared Parakeet Pyrrhura leucotis 5 Klippe Conure Black-capped Parakeet Pyrrhura rupicola 5,5 Lysbuget Conure Maroon-tailed Parakeet Pyrrhura m. berlepschi 5,5 Monagas Conure Venezuelan Parakeet Pyrrhura emma 5 Pacific Sorthalet Conure Maroon-tailed Parakeet Pyrrhura m. pacifica 5,5 Perle Conure Pearly Parakeet Pyrrhura lepida 5,5 Peru Conure Wavy-Breasted Conure Pyrrhura peruviana 5 Rosaisset Conure Rose-fronted Parakeet Pyrrhura roseifrons 5,5 Rødbuget Conure Reddish-bellied Parakeet Pyrrhura frontalis 5,5 Rødisset Conure Rose-crowned Parakeet Pyrrhura rhodocephala 5,5 Sandia Klippe Conure Black-capped Parakeet Pyrrhura r. sandia 5,5 Sorthalet Conure Maroon-tailed Parakeet Pyrrhura melanura 5,5 Venezuela Conure (Emma Conure) Venezuelan Parakeet Pyrrhura emma 5 Kilehaleparakitter Aztekaratinga Aztec Parakeet Aratinga astec 5,5 Blåkindet Aratinga Blue-crowned Parakeet Aratinga acuticaudata 6,5 Brunstrubet Aratinga Brown-throated Parakeet -

TRAFFIC Bird’S-Eye View: REPORT Lessons from 50 Years of Bird Trade Regulation & Conservation in Amazon Countries
TRAFFIC Bird’s-eye view: REPORT Lessons from 50 years of bird trade regulation & conservation in Amazon countries DECEMBER 2018 Bernardo Ortiz-von Halle About the author and this study: Bernardo Ortiz-von Halle, a biologist and TRAFFIC REPORT zoologist from the Universidad del Valle, Cali, Colombia, has more than 30 years of experience in numerous aspects of conservation and its links to development. His decades of work for IUCN - International Union for Conservation of Nature and TRAFFIC TRAFFIC, the wildlife trade monitoring in South America have allowed him to network, is a leading non-governmental organization working globally on trade acquire a unique outlook on the mechanisms, in wild animals and plants in the context institutions, stakeholders and challenges facing of both biodiversity conservation and the conservation and sustainable use of species sustainable development. and ecosystems. Developing a critical perspective The views of the authors expressed in this of what works and what doesn’t to achieve lasting conservation goals, publication do not necessarily reflect those Bernardo has put this expertise within an historic framework to interpret of TRAFFIC, WWF, or IUCN. the outcomes of different wildlife policies and actions in South America, Reproduction of material appearing in offering guidance towards solutions that require new ways of looking at this report requires written permission wildlife trade-related problems. Always framing analysis and interpretation from the publisher. in the midst of the socioeconomic and political frameworks of each South The designations of geographical entities in American country and in the region as a whole, this work puts forward this publication, and the presentation of the conclusions and possible solutions to bird trade-related issues that are material, do not imply the expression of any linked to global dynamics, especially those related to wildlife trade. -

Genetic Diversity and Population Structure of Two Endangered Neotropical Parrots Inform in Situ and Ex Situ Conservation Strategies
diversity Article Genetic Diversity and Population Structure of Two Endangered Neotropical Parrots Inform In Situ and Ex Situ Conservation Strategies Carlos I. Campos 1 , Melinda A. Martinez 1, Daniel Acosta 1, Jose A. Diaz-Luque 2, Igor Berkunsky 3 , Nadine L. Lamberski 4, Javier Cruz-Nieto 5 , Michael A. Russello 6 and Timothy F. Wright 1,* 1 Department of Biology, New Mexico State University, Las Cruces, NM 88003, USA; [email protected] (C.I.C.); [email protected] (M.A.M.); [email protected] (D.A.) 2 Fundación CLB (FPCILB), Estación Argentina, Calle Fermín Rivero 3460, Santa Cruz de la Sierra, Bolivia; [email protected] 3 Instituto Multidisciplinario sobre Ecosistemas y Desarrollo Sustenable, CONICET-Universidad Nacional del Centro de la Provincia de Buenos Aires, Tandil 7000, Argentina; [email protected] 4 San Diego Zoo Wildlife Alliance, San Diego, CA 92027, USA; [email protected] 5 Organización Vida Silvestre A.C. (OVIS), San Pedro Garza Garciá 66260, Mexico; [email protected] 6 Department of Biology, University of British Columbia, Kelowna, BC V1V 1V7, Canada; [email protected] * Correspondence: [email protected] Abstract: A key aspect in the conservation of endangered populations is understanding patterns of Citation: Campos, C.I.; Martinez, genetic variation and structure, which can provide managers with critical information to support M.A.; Acosta, D.; Diaz-Luque, J.A.; evidence-based status assessments and management strategies. This is especially important for Berkunsky, I.; Lamberski, N.L.; species with small wild and larger captive populations, as found in many endangered parrots. We Cruz-Nieto, J.; Russello, M.A.; Wright, used genotypic data to assess genetic variation and structure in wild and captive populations of T.F. -

Phylogeography of the Military Macaw (Ara Militaris) and the Great Green Macaw (A
The Wilson Journal of Ornithology 127(4):661–669, 2015 PHYLOGEOGRAPHY OF THE MILITARY MACAW (ARA MILITARIS) AND THE GREAT GREEN MACAW (A. AMBIGUUS) BASED ON MTDNA SEQUENCE DATA JESSICA R. EBERHARD,1,5 EDUARDO E. IÑIGO-ELIAS,2 ERNESTO ENKERLIN-HOEFLICH,3 AND E. PAÙL CUN4 ABSTRACT.—The Military Macaw (Ara militaris) and the Great Green Macaw (A. ambiguus) are species whose close relationship is reflected in their morphological similarity as well as their geographic ranges. Military Macaws have a disjunct distribution, found in Mexico as well as several areas in South America, while Great Green Macaws have two or more disjunct populations from Honduras to eastern Ecuador. We used mitochondrial sequence data to examine the phylogenetic relationships between these two species, and also among representative samples across their ranges. Our data clearly support recognition of the two species as being distinct evolutionary lineages, and while we found significant phylogeographic structure within A. militaris (between samples collected in eastern and western Mexico), we did not find any evidence of lineage divergence between A. ambiguus from Costa Rica and Ecuador. Received 12 December 2014. Accepted 30 May 2015. Key words: disjunct distribution, Great Green Macaw, Military Macaw, phylogeny, phylogeography. The Military Macaw (Ara militaris) and the South America, primarily east of the Andes from Great Green Macaw (A. ambiguus), sometimes northwestern Colombia and northwestern Vene- named Buffon’s Macaw, are both large macaws zuela to north-western Argentina (Ridgway 1916; that are closely related and possibly conspecific Chapman 1917; Alvarez del Toro 1980; Ridgely (Fjeldså et al. 1987, Collar et al. -

Parrot Brochure
COMMON MEDICAL PROPER HOUSING COMPANION DISEASES PARROTS: 1.) Nutritional deficiencies - A variety of ocular, nasal, respiratory, reproductive LARGE & SMALL and skin disorders caused by chronically improper diets. 2.) Feather picking - A behavioral disorder, sometimes secondary to a primary medical problem, where the bird self-mutilates by picking out its own Maecenas feathers. It is most often due to depression from lack of mental Proper housing for a macaw and other large birds stimulation or companionship and more Finding the right parrot cage for your feathered commonly seen in larger species. friend depends on the size and needs of your Purchasing your pet birds only in pairs bird. For example, while a parakeet needs a can help prevent this disorder smaller cage that can sit on a counter-top or from developing." table; the macaw needs a HUGE cage practically 3.) Bumblefoot - All caged birds are the size of a small room! It is always safest to “go susceptible to developing “bumblefoot" big.” Avoid galvanized metal wiring due to the or pododermatitis. This disease manifests potential for lead poisoning, and clean the itself as blisters and infections of the feet substrate on the bottom of the cage daily to caused by dirty perches or perches that weekly. Birds are messy creatures that love to are all the same size, shape and made of dive into their food bowls! Perches should vary the same material. i.e. smooth wood. in size, shape and material; including various How best to care for these diverse woods, sand paper and cloth. Clean perches and colorful birds and to ensure regularly to prevent diseases of the feet. -

Juan Cristóbal Gundlach's Collections of Puerto Rican Birds with Special
ZOBODAT - www.zobodat.at Zoologisch-Botanische Datenbank/Zoological-Botanical Database Digitale Literatur/Digital Literature Zeitschrift/Journal: Zoosystematics and Evolution Jahr/Year: 2015 Band/Volume: 91 Autor(en)/Author(s): Frahnert Sylke, Roman Rafela Aguilera, Eckhoff Pascal, Wiley James W. Artikel/Article: Juan Cristóbal Gundlach’s collections of Puerto Rican birds with special regard to types 177-189 Creative Commons Attribution 4.0 licence (CC-BY); original download https://pensoft.net/journals Zoosyst. Evol. 91 (2) 2015, 177–189 | DOI 10.3897/zse.91.5550 museum für naturkunde Juan Cristóbal Gundlach’s collections of Puerto Rican birds with special regard to types Sylke Frahnert1, Rafaela Aguilera Román2, Pascal Eckhoff1, James W. Wiley3 1 Museum für Naturkunde, Leibniz-Institut für Evolutions- und Biodiversitätsforschung, Invalidenstraße 43, D-10115 Berlin, Germany 2 Instituto de Ecología y Sistemática, La Habana, Cuba 3 PO Box 64, Marion Station, Maryland 21838-0064, USA http://zoobank.org/B4932E4E-5C52-427B-977F-83C42994BEB3 Corresponding author: Sylke Frahnert ([email protected]) Abstract Received 1 July 2015 The German naturalist Juan Cristóbal Gundlach (1810–1896) conducted, while a resident Accepted 3 August 2015 of Cuba, two expeditions to Puerto Rico in 1873 and 1875–6, where he explored the Published 3 September 2015 southwestern, western, and northeastern regions of this island. Gundlach made repre sentative collections of the island’s fauna, which formed the nucleus of the first natural Academic editor: history museums in Puerto Rico. When the natural history museums closed, only a few Peter Bartsch specimens were passed to other institutions, including foreign museums. None of Gund lach’s and few of his contemporaries’ specimens have survived in Puerto Rico. -

A Courting Behavioral Study on a Hyacinth Macaw (Anodorhynchus Hyacinthinus) Pair
The Pegasus Review: UCF Undergraduate Research Journal (URJ) Volume 12 Issue 1 Article 2 2020 A Courting Behavioral Study on a Hyacinth Macaw (Anodorhynchus hyacinthinus) Pair Pamela Mulkay University of Central Florida Find similar works at: https://stars.library.ucf.edu/urj University of Central Florida Libraries http://library.ucf.edu This Article is brought to you for free and open access by the Office of Undergraduate Research at STARS. It has been accepted for inclusion in The Pegasus Review: UCF Undergraduate Research Journal (URJ) by an authorized editor of STARS. For more information, please contact [email protected]. Recommended Citation Mulkay, Pamela (2020) "A Courting Behavioral Study on a Hyacinth Macaw (Anodorhynchus hyacinthinus) Pair," The Pegasus Review: UCF Undergraduate Research Journal (URJ): Vol. 12 : Iss. 1 , Article 2. Available at: https://stars.library.ucf.edu/urj/vol12/iss1/2 Mulkay: A Courting Behavioral Study on a Hyacinth Macaw Published 9-17 Vol. 12.1: April 8, 2020 THE PEGASUS REVIEW: UNIVERSITY OF CENTRAL FLORIDA UNDERGRADUATE RESEARCH JOURNAL A Courting Behavioral Study on a Hyacinth Macaw (Anodorhynchus hyacinthinus) Pair By: Pamela Mulkay Faculty Mentor: Frank Logiudice UCF Department of Biology ABSTRACT: This study observes the courtship behaviors of an Anodorhynchus hyacinthinus pair in the Central Florida Zoo and Botanical Gardens in Sanford, Florida. A. hyacinthinus reproductive behaviors occur in four steps in the following order: Allopreening, Cloacal allopreening, Back to Back Copulation Position and finally, Copulation (Schneider 2006). Behavioral observations were taken twice a week for an average of 2 to 3 hours each day for ten weeks. The resulting data was analyzed based on the different actions, types of movement, and types of maintenance observed of the A.