Anaerobic Endosymbiont Generates Energy for Ciliate Host by Denitrification
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Jpn. J. Protozool. 38(2) 171-183
Jpn. J. Protozool. Vol. 38, No. 2. (2005) 171 Review On the origin of mitochondria and Rickettsia-related eukaryotic endo- symbionts B. Franz Lang1*, Henner Brinkmann1, Liisa B. Koski1, Masahiro Fujishima2, Hans-Dieter Görtz3 and Gertraud Burger1 1Program in Evolutionary Biology, Canadian Institute for Advanced Research; Centre Robert Cedergren, Département de Biochimie, Université de Montréal, 2900 Boulevard Edouard- Montpetit, Montréal, Québec, H3T 1J4, Canada. 2Biological Institute, Faculty of Science, Yamaguchi University, Yamaguchi 753-8512, Japan. 3Abteilung Zoologie, Biologisches Institut, Universität Stuttgart, Pfaffenwaldring 57, 70550 Stuttgart, Germany. SUMMARY sence of genes for oxidative phosphorylation, the TCA cycle, and many other metabolic pathways, Resent insights into the origin and early evo- but the presence of several pathogenesis-related lution of mitochondria come from two approaches: genes and a high number of bacterial IS elements. the investigation of mtDNAs from minimally de- Phylogenetic analyses with multiple protein se- rived (primitive) mitochondriate eukaryotes, in quences place H. obtusa basally to the Rickettsia- particular jakobid flagellates, and of genomes from Ehrlichia-Wolbachia assemblage of bacterial intracellular α-proteobacterial symbionts. Of par- pathogens. This leads us to postulate that H. ob- ticular interest in this context is Holospora obtusa, tusa is the closest bacterial relative of mitochon- an intracellular bacterial endosymbiont that resides dria known to date. and replicates in the somatic nucleus of its eu- karyotic host, the ciliate Paramecium caudatum. Currently we have sequenced close to 50% of the INTRODUCTION ~ 1.7 Mbp H. obtusa genome, revealing the ab- One of the major advancements in under- standing eukaryotic evolution was the discovery that mitochondria evolved from an endosymbiotic α-Proteobacterium, and that mitochondrial DNA *Corresponding author (mtDNA) is a relict bacterial genome. -

Sex Is a Ubiquitous, Ancient, and Inherent Attribute of Eukaryotic Life
PAPER Sex is a ubiquitous, ancient, and inherent attribute of COLLOQUIUM eukaryotic life Dave Speijera,1, Julius Lukešb,c, and Marek Eliášd,1 aDepartment of Medical Biochemistry, Academic Medical Center, University of Amsterdam, 1105 AZ, Amsterdam, The Netherlands; bInstitute of Parasitology, Biology Centre, Czech Academy of Sciences, and Faculty of Sciences, University of South Bohemia, 370 05 Ceské Budejovice, Czech Republic; cCanadian Institute for Advanced Research, Toronto, ON, Canada M5G 1Z8; and dDepartment of Biology and Ecology, University of Ostrava, 710 00 Ostrava, Czech Republic Edited by John C. Avise, University of California, Irvine, CA, and approved April 8, 2015 (received for review February 14, 2015) Sexual reproduction and clonality in eukaryotes are mostly Sex in Eukaryotic Microorganisms: More Voyeurs Needed seen as exclusive, the latter being rather exceptional. This view Whereas absence of sex is considered as something scandalous for might be biased by focusing almost exclusively on metazoans. a zoologist, scientists studying protists, which represent the ma- We analyze and discuss reproduction in the context of extant jority of extant eukaryotic diversity (2), are much more ready to eukaryotic diversity, paying special attention to protists. We accept that a particular eukaryotic group has not shown any evi- present results of phylogenetically extended searches for ho- dence of sexual processes. Although sex is very well documented mologs of two proteins functioning in cell and nuclear fusion, in many protist groups, and members of some taxa, such as ciliates respectively (HAP2 and GEX1), providing indirect evidence for (Alveolata), diatoms (Stramenopiles), or green algae (Chlor- these processes in several eukaryotic lineages where sex has oplastida), even serve as models to study various aspects of sex- – not been observed yet. -

BMC Research Notes Biomed Central
BMC Research Notes BioMed Central Short Report Open Access A split and rearranged nuclear gene encoding the iron-sulfur subunit of mitochondrial succinate dehydrogenase in Euglenozoa Ryan MR Gawryluk and Michael W Gray* Address: Department of Biochemistry and Molecular Biology, Dalhousie University, Halifax, Nova Scotia B3H 1X5, Canada Email: Ryan MR Gawryluk - [email protected]; Michael W Gray* - [email protected] * Corresponding author Published: 3 February 2009 Received: 18 December 2008 Accepted: 3 February 2009 BMC Research Notes 2009, 2:16 doi:10.1186/1756-0500-2-16 This article is available from: http://www.biomedcentral.com/1756-0500/2/16 © 2009 Gray et al; licensee BioMed Central Ltd. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. Abstract Background: Analyses based on phylogenetic and ultrastructural data have suggested that euglenids (such as Euglena gracilis), trypanosomatids and diplonemids are members of a monophyletic lineage termed Euglenozoa. However, many uncertainties are associated with phylogenetic reconstructions for ancient and rapidly evolving groups; thus, rare genomic characters become increasingly important in reinforcing inferred phylogenetic relationships. Findings: We discovered that the iron-sulfur subunit (SdhB) of mitochondrial succinate dehydrogenase is encoded by a split and rearranged nuclear gene in Euglena gracilis and trypanosomatids, an example of a rare genomic character. The two subgenic modules are transcribed independently and the resulting mRNAs appear to be independently translated, with the two protein products imported into mitochondria, based on the presence of predicted mitochondrial targeting peptides. -

PROTISTAS MARINOS Viviana A
PROTISTAS MARINOS Viviana A. Alder INTRODUCCIÓN plantas y animales. Según este esquema básico, a las plantas les correspondían las características de En 1673, el editor de Philosophical Transac- ser organismos sésiles con pigmentos fotosinté- tions of the Royal Society of London recibió una ticos para la síntesis de las sustancias esenciales carta del anatomista Regnier de Graaf informan- para su metabolismo a partir de sustancias inor- do que un comerciante holandés, Antonie van gánicas (nutrición autótrofa), y de poseer células Leeuwenhoek, había “diseñado microscopios rodeadas por paredes de celulosa. En oposición muy superiores a aquéllos que hemos visto has- a las plantas, les correspondía a los animales los ta ahora”. Van Leeuwenhoek vendía lana, algo- atributos de tener motilidad activa y de carecer dón y otros materiales textiles, y se había visto tanto de pigmentos fotosintéticos (debiendo por en la necesidad de mejorar las lentes de aumento lo tanto procurarse su alimento a partir de sustan- que comúnmente usaba para contar el número cias orgánicas sintetizadas por otros organismos) de hebras y evaluar la calidad de fibras y tejidos. como de paredes celulósicas en sus células. Así fue que construyó su primer microscopio de Es a partir de los estudios de Georg Gol- lente única: simple, pequeño, pero con un poder dfuss (1782-1848) que estos diminutos organis- de magnificación de hasta 300 aumentos (¡diez mos, invisibles a ojo desnudo, comienzan a ser veces más que sus precursores!). Este magnífico clasificados como plantas primarias -

The Revised Classification of Eukaryotes
See discussions, stats, and author profiles for this publication at: https://www.researchgate.net/publication/231610049 The Revised Classification of Eukaryotes Article in Journal of Eukaryotic Microbiology · September 2012 DOI: 10.1111/j.1550-7408.2012.00644.x · Source: PubMed CITATIONS READS 961 2,825 25 authors, including: Sina M Adl Alastair Simpson University of Saskatchewan Dalhousie University 118 PUBLICATIONS 8,522 CITATIONS 264 PUBLICATIONS 10,739 CITATIONS SEE PROFILE SEE PROFILE Christopher E Lane David Bass University of Rhode Island Natural History Museum, London 82 PUBLICATIONS 6,233 CITATIONS 464 PUBLICATIONS 7,765 CITATIONS SEE PROFILE SEE PROFILE Some of the authors of this publication are also working on these related projects: Biodiversity and ecology of soil taste amoeba View project Predator control of diversity View project All content following this page was uploaded by Smirnov Alexey on 25 October 2017. The user has requested enhancement of the downloaded file. The Journal of Published by the International Society of Eukaryotic Microbiology Protistologists J. Eukaryot. Microbiol., 59(5), 2012 pp. 429–493 © 2012 The Author(s) Journal of Eukaryotic Microbiology © 2012 International Society of Protistologists DOI: 10.1111/j.1550-7408.2012.00644.x The Revised Classification of Eukaryotes SINA M. ADL,a,b ALASTAIR G. B. SIMPSON,b CHRISTOPHER E. LANE,c JULIUS LUKESˇ,d DAVID BASS,e SAMUEL S. BOWSER,f MATTHEW W. BROWN,g FABIEN BURKI,h MICAH DUNTHORN,i VLADIMIR HAMPL,j AARON HEISS,b MONA HOPPENRATH,k ENRIQUE LARA,l LINE LE GALL,m DENIS H. LYNN,n,1 HILARY MCMANUS,o EDWARD A. D. -

Role of Lipids and Fatty Acids in Stress Tolerance in Cyanobacteria
Acta Protozool. (2002) 41: 297 - 308 Review Article Role of Lipids and Fatty Acids in Stress Tolerance in Cyanobacteria Suresh C. SINGH, Rajeshwar P. SINHA and Donat-P. HÄDER Institut für Botanik und Pharmazeutische Biologie, Friedrich-Alexander-Universität, Erlangen, Germany Summary. Lipids are the most effective source of storage energy, function as insulators of delicate internal organs and hormones and play an important role as the structural constituents of most of the cellular membranes. They also have a vital role in tolerance to several physiological stressors in a variety of organisms including cyanobacteria. The mechanism of desiccation tolerance relies on phospholipid bilayers which are stabilized during water stress by sugars, especially by trehalose. Unsaturation of fatty acids also counteracts water or salt stress. Hydrogen atoms adjacent to olefinic bonds are susceptible to oxidative attack. Lipids are rich in these bonds and are a primary target for oxidative reactions. Lipid oxidation is problematic as enzymes do not control many oxidative chemical reactions and some of the products of the attack are highly reactive species that modify proteins and DNA. This review deals with the role of lipids and fatty acids in stress tolerance in cyanobacteria. Key words: cyanobacteria, desiccation, fatty acids, lipids, salinity, temperature stress. INTRODUCTION The cyanobacteria such as Spirulina and Nostoc have been used as a source of protein and vitamin for Cyanobacteria are gram-negative photoautotrophic humans and animals (Ciferri 1983, Kay 1991, Gao 1998, prokaryotes having ´higher plant-type‘ oxygenic photo- Takenaka et al. 1998). Spirulina has an unusually high synthesis (Stewart 1980, Sinha and Häder 1996a). -

Statistics of Exon Lengths in Fungi
The Open Bioinformatics Journal, 2010, 4, 31-40 31 Open Access Statistics of Exon Lengths in Fungi Alexander Kaplunovsky1, David Zabrodsky2, Zeev Volkovich2, Anatoliy Ivashchenko3 and Alexander Bolshoy*,1 1Department of Evolutionary and Environmental Biology and Genome Diversity Center at the Institute of Evolution, University of Haifa, Israel 2Department of Software Engineering, ORT Braude College, Karmiel, Israel 3Department of Biotechnology, Biochemistry, Plant Physiology at the Al-Farabi Kazakh National University, Kazakhstan Abstract: The exon-intron structures of fungi genes are quite different from each other, and the evolution of such struc- tures raises many questions. We tried to address some of these questions with an accent on methods of revealing evolu- tionary factors based on the analysis of gene exon-intron structures using statistical analysis. Taking whole genomes of fungi, we went through all the protein-coding genes in each chromosome separately and calculated the portion of intron- containing genes and average values of the net length of all the exons in a gene, the number of the exons, and the average length of an exon. We found striking similarities between all of these average properties of chromosomes of the same spe- cies and significant differences between properties of the chromosomes belonging to species of different divisions (Phyla) of the kingdom of Fungi. Comparing those chromosomal and genomic averages, we have developed a technique of clus- tering based on characteristics of the exon-intron structure. This technique of clustering separates different fungi species, grouping them according to Fungi taxonomy. The main conclusion of this article is that the statistical properties of exon- intron organizations of genes are the genome-specific features preserved by evolutionary processes. -

Morphology and Phylogeny of Three Trachelocercids (Protozoa
Zoological Journal of the Linnean Society, 2016, 177 , 306–319. With 9 figures Morphology and phylogeny of three trachelocercids (Protozoa, Ciliophora, Karyorelictea), with description of two new species and insight into the evolution of the family Trachelocercidae YING YAN1,2, YUAN XU1*, SALEH A. AL-FARRAJ3, KHALED A. S. AL-RASHEID3 and WEIBO SONG2 1State Key Laboratory of Estuarine and Coastal Research, East China Normal University, Shanghai 200062, China 2Institute of Evolution & Marine Biodiversity, Ocean University of China, Qingdao 266003, China 3Zoology Department, College of Science, King Saud University, Riyadh 11451, Saudi Arabia Received 5 August 2015; revised 23 September 2015; accepted for publication 24 September 2015 Although trachelocercid ciliates are common in marine sandy intertidal zones, methodological difficulties mean that their biodiversity and evolutionary relationships have not been well documented. This paper investigates the morphology and infraciliature of two novel Trachelolophos and one rarely known form, Tracheloraphis similis Raikov and Kovaleva, 1968, collected from the coastal waters of southern and eastern China. The small subunit (SSU) rRNA gene sequences of two of the species are presented, allowing the phylogenetic position of the genus Trachelolophos to be revealed for the first time. Phylogenetic analyses based on SSU rRNA gene sequences indicate that Trachelolophos branches with Kovalevaia and forms a sister clade with the group including Prototrachelocerca, Trachelocerca and Tracheloraphis. The monophyly of Trachelocerca is not rejected by the approximately unbiased (AU) test (P = 0.209, > 0.05) but that of Tracheloraphis is rejected (P = 3e-033, < 0.05). The newly sequenced genus Trachelolophos, and recent studies on the morphology and phylogeny of the family Trachelocercidae, suggest two new hypotheses about the evolution of the seven genera within Trachelocercidae, based on either infraciliature or molecular evidence. -

Origin and Diversification of Eukaryotes
MI66CH20-Katz ARI 21 June 2012 17:22 V I E E W R S Review in Advance first posted online on July 9, 2012. (Changes may still occur before final publication E online and in print.) I N C N A D V A Origin and Diversification of Eukaryotes Laura A. Katz Department of Biological Sciences, Smith College, Northampton, Massachusetts 01063; email: [email protected] Program in Organismic and Evolutionary Biology, University of Massachusetts, Amherst, Massachusetts 01003 Annu. Rev. Microbiol. 2012. 66:411–27 Keywords The Annual Review of Microbiology is online at eukaryotic diversity, protists, tree of life, nucleus, cytoskeleton, micro.annualreviews.org mitochondria This article’s doi: by SMITH COLLEGE on 08/12/12. For personal use only. 10.1146/annurev-micro-090110-102808 Abstract Copyright c 2012 by Annual Reviews. The bulk of the diversity of eukaryotic life is microbial. Although the larger All rights reserved Annu. Rev. Microbiol. 2012.66. Downloaded from www.annualreviews.org eukaryotes—namely plants, animals, and fungi—dominate our visual land- 0066-4227/12/1013-0411$20.00 scapes, microbial lineages compose the greater part of both genetic diversity and biomass, and contain many evolutionary innovations. Our understand- ing of the origin and diversification of eukaryotes has improved substan- tially with analyses of molecular data from diverse lineages. These data have provided insight into the nature of the genome of the last eukaryotic com- mon ancestor (LECA). Yet, the origin of key eukaryotic features, namely the nucleus and cytoskeleton, remains poorly understood. In contrast, the past decades have seen considerable refinement in hypotheses on the major branching events in the evolution of eukaryotic diversity. -

Organellar Genome Evolution in Red Algal Parasites: Differences in Adelpho- and Alloparasites
University of Rhode Island DigitalCommons@URI Open Access Dissertations 2017 Organellar Genome Evolution in Red Algal Parasites: Differences in Adelpho- and Alloparasites Eric Salomaki University of Rhode Island, [email protected] Follow this and additional works at: https://digitalcommons.uri.edu/oa_diss Recommended Citation Salomaki, Eric, "Organellar Genome Evolution in Red Algal Parasites: Differences in Adelpho- and Alloparasites" (2017). Open Access Dissertations. Paper 614. https://digitalcommons.uri.edu/oa_diss/614 This Dissertation is brought to you for free and open access by DigitalCommons@URI. It has been accepted for inclusion in Open Access Dissertations by an authorized administrator of DigitalCommons@URI. For more information, please contact [email protected]. ORGANELLAR GENOME EVOLUTION IN RED ALGAL PARASITES: DIFFERENCES IN ADELPHO- AND ALLOPARASITES BY ERIC SALOMAKI A DISSERTATION SUBMITTED IN PARTIAL FULFILLMENT OF THE REQUIREMENTS FOR THE DEGREE OF DOCTOR OF PHILOSOPHY IN BIOLOGICAL SCIENCES UNIVERSITY OF RHODE ISLAND 2017 DOCTOR OF PHILOSOPHY DISSERTATION OF ERIC SALOMAKI APPROVED: Dissertation Committee: Major Professor Christopher E. Lane Jason Kolbe Tatiana Rynearson Nasser H. Zawia DEAN OF THE GRADUATE SCHOOL UNIVERSITY OF RHODE ISLAND 2017 ABSTRACT Parasitism is a common life strategy throughout the eukaryotic tree of life. Many devastating human pathogens, including the causative agents of malaria and toxoplasmosis, have evolved from a photosynthetic ancestor. However, how an organism transitions from a photosynthetic to a parasitic life history strategy remains mostly unknown. Parasites have independently evolved dozens of times throughout the Florideophyceae (Rhodophyta), and often infect close relatives. This framework enables direct comparisons between autotrophs and parasites to investigate the early stages of parasite evolution. -

10531 2007 9250 Article-Web 1..16
Biodivers Conserv (2008) 17:261–276 DOI 10.1007/s10531-007-9250-y ORIGINAL PAPER “Missing” protists: a molecular prospective Slava Epstein · PuriWcación López-García Received: 13 March 2007 / Accepted: 30 May 2007 / Published online: 24 October 2007 © Springer Science+Business Media B.V. 2007 Abstract Molecular ecology methods based on 18S rRNA ampliWcation and sequencing have revealed an astounding diversity of microbial eukaryotes in every environment sam- pled so far. This is certainly true of new species and genera, as essentially every new survey discovers a wealth of novel diversity at this level. This is almost certain for taxa that are higher in taxonomic hierarchy, as many molecular surveys reported novel clades within established protistan phyla, with some of these clades repeatedly conWrmed by subsequent studies. It may also be that the molecular approaches discovered several lineages of the highest taxonomic order, but this claim has not been vigorously veriWed as yet. Overall, the Weld of protistan diversity remains in its infancy. The true scale of this diversity is unknown, and so are the distribution of this diversity, its patterns, spatial and temporal dynamics, and ecological role. The sampled diversity appears to be just the tip of the ice- berg, and this oVers outstanding opportunities for microbial discovery for the purposes of both basic and applied research. Keywords 18S rRNA · Cryptic species · Eukaryotic phylogeny · Molecular ecology · Species richness Introduction Molecular analyses based on the rRNA approach have become an aVordable and practical tool that is used routinely to study microbial diversity in environmental samples. This type Special Issue: Protist diversity and geographic distribution. -
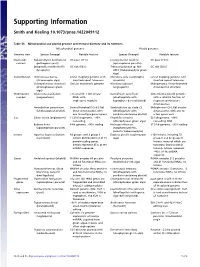
Supporting Information
Supporting Information Smith and Keeling 10.1073/pnas.1422049112 Table S1. Mitochondrial and plastid genome architectural diversity and its extremes Mitochondrial genomes Plastid genomes Genomic trait Species (lineage) Notable feature Species (lineage) Notable feature Nucleotide Nakaseomyces bacillisporus GC poor (11%) Leucocytozoon caulleryi GC poor (15%) content (pathogenic yeast) (apicomplexan parasite) Selaginella moellendorffii GC rich (68%) Trebouxiophyceae sp. MX- GC rich (58%) (lycophyte) AZ01 (trebouxiophyte green alga) Conformation Ochromonas danica Linear-mapping genome with Chromera velia (autotrophic Linear-mapping genome with (stramenopile alga) inverted-repeat telomeres alveolate) inverted repeat telomeres Chlamydomonas moewusii Circular monomeric genome Nicotiana tabacum Multigenomic, linear-branched (chlorophycean green (angiosperm) chromosomal structure alga) Chromosome Diplonema papillatum >75 small (6–7 kb) circular Karlodinium veneficum Conventional plastid genome number (euglenozoan) DNAs with (dinoflagellate with with a satellite fraction of single-gene modules haptophyte-derived plastid) fragmented miniature chromosomes Amoebidium parasiticum Several hundred (0.3–8.3 kb) Symbiodinium sp. clade C3 Multiple mini (2–3 kb) circular (ichthyosporean protist) linear chromosomes with (dinoflagellate with chromosomes with one to one to multiple genes apiece peridinin-containing plastid) a few genes each Size Silene conica (angiosperm) 11,318-kb genome, ∼99% Floydiella terrestris 521 kb genome, ∼80% noncoding (chlorophycean green alga) noncoding DNA Babesia bovis 6-kb genome, >90% coding Helicosporidium sp. 37.5 kb genome, ∼95% coding (apicomplexan parasite) (nonphotosynthetic, parasitic trebouxiophyte) Introns Agaricus bisporus (button 43 group I and 3 group II Euglena gracilis (euglenozoan >150 introns, including 72 mushroom) introns distributed in 8 of 15 alga) group II and 46 group III protein-coding genes. introns, many of which are Introns represent 45% of the twintrons (introns within mtDNA sequence introns).