Infrared Target and Background Radiometric Measurements-Concepts, Units, and Techniques
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
Plant Lighting Basics and Applications
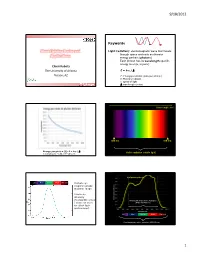
9/18/2012 Keywords Plant Lighting Basics and Light (radiation): electromagnetic wave that travels through space and exits as discrete Applications energy packets (photons) Each photon has its wavelength-specific energy level (E, in joule) Chieri Kubota The University of Arizona E = h·c / Tucson, AZ E: Energy per photon (joule per photon) h: Planck’s constant c: speed of light Greensys 2011, Greece : wavelength (meter) Wavelength (nm) 380 nm 780 nm Energy per photon [J]: E = h·c / Visible radiation (visible light) mole photons = 6.02 x 1023 photons Leaf photosynthesis UV Blue Green Red Far red Human eye response peaks at green range. Luminous intensity (footcandle or lux Photosynthetically Active Radiation ) does not work (PAR, 400-700 nm) for plant light environment. UV Blue Green Red Far red Plant biologically active radiation (300-800 nm) 1 9/18/2012 Chlorophyll a Phytochrome response Chlorophyll b UV Blue Green Red Far red Absorption spectra of isolated chlorophyll Absorptance 400 500 600 700 Wavelength (nm) Daily light integral (DLI or Daily Light Unit and Terminology PPF) Radiation Photons Visible light •Total amount of photosynthetically active “Base” unit Energy (J) Photons (mol) Luminous intensity (cd) radiation (400‐700 nm) received per sq Flux [total amount received Radiant flux Photon flux Luminous flux meter per day or emitted per time] (J s-1) or (W) (mol s-1) (lm) •Unit: mole per sq meter per day (mol m‐2 d‐1) Flux density [total amount Radiant flux density Photon flux (density) Illuminance, • Under optimal conditions, plant growth is received per area per time] (W m-2) (mol m-2 s-1) Luminous flux density highly correlated with DLI. -

Spectral Reflectance and Emissivity of Man-Made Surfaces Contaminated with Environmental Effects
Optical Engineering 47͑10͒, 106201 ͑October 2008͒ Spectral reflectance and emissivity of man-made surfaces contaminated with environmental effects John P. Kerekes, MEMBER SPIE Abstract. Spectral remote sensing has evolved considerably from the Rochester Institute of Technology early days of airborne scanners of the 1960’s and the first Landsat mul- Chester F. Carlson Center for Imaging Science tispectral satellite sensors of the 1970’s. Today, airborne and satellite 54 Lomb Memorial Drive hyperspectral sensors provide images in hundreds of contiguous narrow Rochester, New York 14623 spectral channels at spatial resolutions down to meter scale and span- E-mail: [email protected] ning the optical spectral range of 0.4 to 14 m. Spectral reflectance and emissivity databases find use not only in interpreting these images but also during simulation and modeling efforts. However, nearly all existing Kristin-Elke Strackerjan databases have measurements of materials under pristine conditions. Aerospace Engineering Test Establishment The work presented extends these measurements to nonpristine condi- P.O. Box 6550 Station Forces tions, including materials contaminated with sand and rain water. In par- Cold Lake, Alberta ticular, high resolution spectral reflectance and emissivity curves are pre- T9M 2C6 Canada sented for several man-made surfaces ͑asphalt, concrete, roofing shingles, and vehicles͒ under varying amounts of sand and water. The relationship between reflectance and area coverage of the contaminant Carl Salvaggio, MEMBER SPIE is reported and found to be linear or nonlinear, depending on the mate- Rochester Institute of Technology rials and spectral region. In addition, new measurement techniques are Chester F. Carlson Center for Imaging Science presented that overcome limitations of existing instrumentation and labo- 54 Lomb Memorial Drive ratory settings. -

Fundametals of Rendering - Radiometry / Photometry
Fundametals of Rendering - Radiometry / Photometry “Physically Based Rendering” by Pharr & Humphreys •Chapter 5: Color and Radiometry •Chapter 6: Camera Models - we won’t cover this in class 782 Realistic Rendering • Determination of Intensity • Mechanisms – Emittance (+) – Absorption (-) – Scattering (+) (single vs. multiple) • Cameras or retinas record quantity of light 782 Pertinent Questions • Nature of light and how it is: – Measured – Characterized / recorded • (local) reflection of light • (global) spatial distribution of light 782 Electromagnetic spectrum 782 Spectral Power Distributions e.g., Fluorescent Lamps 782 Tristimulus Theory of Color Metamers: SPDs that appear the same visually Color matching functions of standard human observer International Commision on Illumination, or CIE, of 1931 “These color matching functions are the amounts of three standard monochromatic primaries needed to match the monochromatic test primary at the wavelength shown on the horizontal scale.” from Wikipedia “CIE 1931 Color Space” 782 Optics Three views •Geometrical or ray – Traditional graphics – Reflection, refraction – Optical system design •Physical or wave – Dispersion, interference – Interaction of objects of size comparable to wavelength •Quantum or photon optics – Interaction of light with atoms and molecules 782 What Is Light ? • Light - particle model (Newton) – Light travels in straight lines – Light can travel through a vacuum (waves need a medium to travel in) – Quantum amount of energy • Light – wave model (Huygens): electromagnetic radiation: sinusiodal wave formed coupled electric (E) and magnetic (H) fields 782 Nature of Light • Wave-particle duality – Light has some wave properties: frequency, phase, orientation – Light has some quantum particle properties: quantum packets (photons). • Dimensions of light – Amplitude or Intensity – Frequency – Phase – Polarization 782 Nature of Light • Coherence - Refers to frequencies of waves • Laser light waves have uniform frequency • Natural light is incoherent- waves are multiple frequencies, and random in phase. -

TIE-35: Transmittance of Optical Glass
DATE October 2005 . PAGE 1/12 TIE-35: Transmittance of optical glass 0. Introduction Optical glasses are optimized to provide excellent transmittance throughout the total visible range from 400 to 800 nm. Usually the transmittance range spreads also into the near UV and IR regions. As a general trend lowest refractive index glasses show high transmittance far down to short wavelengths in the UV. Going to higher index glasses the UV absorption edge moves closer to the visible range. For highest index glass and larger thickness the absorption edge already reaches into the visible range. This UV-edge shift with increasing refractive index is explained by the general theory of absorbing dielectric media. So it may not be overcome in general. However, due to improved melting technology high refractive index glasses are offered nowadays with better blue-violet transmittance than in the past. And for special applications where best transmittance is required SCHOTT offers improved quality grades like for SF57 the grade SF57 HHT. The aim of this technical information is to give the optical designer a deeper understanding on the transmittance properties of optical glass. 1. Theoretical background A light beam with the intensity I0 falls onto a glass plate having a thickness d (figure 1-1). At the entrance surface part of the beam is reflected. Therefore after that the intensity of the beam is Ii0=I0(1-r) with r being the reflectivity. Inside the glass the light beam is attenuated according to the exponential function. At the exit surface the beam intensity is I i = I i0 exp(-kd) [1.1] where k is the absorption constant. -

Reflectance and Transmittance Sphere
Reflectance and Transmittance Sphere Spectral Evolution four inch portable reflectance/ transmittance sphere is ideal for measuring vegetation physiological states SPECTRAL EVOLUTION offers a portable, compact four inch reflectance/transmittance (R/T) integrating sphere for measuring the reflectance and transmittance of vegetation. The 4 inch R/T sphere is lightweight and portable so you can take it into the field for in situ meas- urements, delivered with a stand as well as a ¼-20 mount for use with tripods. When used with a SPECTRAL EVOLUTION spectrometer or spectroradiometer such as the PSR+, RS-3500, RS- 5400, SR-6500 or RS-8800, it delivers detailed information for measurements of transmittance and reflectance modes. Two varying light intensity levels (High and Low) allow for a broader range of samples to be measured. Light reflected or transmitted from a sample in a sphere is integrated over a full hemisphere, with measurements insensitive to sample anisotropic directional reflectance (transmittance). This provides repeatable measurements of a variety of samples. The 4 inch portable R/T sphere can be used in vegetation studies such as, deriving absorbance characteristics, and estimating the leaf area index (LAI) from radiation reflected from a canopy surface. The sphere allows researchers to limit destructive sampling and provides timely data acquisition of key material samples. The R/T sphere allows you to collect all diffuse light reflected from a sample – measuring total hemispherical reflectance. You can measure reflectance and transmittance, and calculate absorption. You can select the bulb inten- sity level that suits your sample. A white reference is provided for both reflectance and transmittance measurements. -

About SI Units
Units SI units: International system of units (French: “SI” means “Système Internationale”) The seven SI base units are: Mass: kg Defined by the prototype “standard kilogram” located in Paris. The standard kilogram is made of Pt metal. Length: m Originally defined by the prototype “standard meter” located in Paris. Then defined as 1,650,763.73 wavelengths of the orange-red radiation of Krypton86 under certain specified conditions. (Official definition: The distance traveled by light in vacuum during a time interval of 1 / 299 792 458 of a second) Time: s The second is defined as the duration of a certain number of oscillations of radiation coming from Cs atoms. (Official definition: The second is the duration of 9,192,631,770 periods of the radiation of the hyperfine- level transition of the ground state of the Cs133 atom) Current: A Defined as the current that causes a certain force between two parallel wires. (Official definition: The ampere is that constant current which, if maintained in two straight parallel conductors of infinite length, of negligible circular cross-section, and placed 1 meter apart in vacuum, would produce between these conductors a force equal to 2 × 10-7 Newton per meter of length. Temperature: K One percent of the temperature difference between boiling point and freezing point of water. (Official definition: The Kelvin, unit of thermodynamic temperature, is the fraction 1 / 273.16 of the thermodynamic temperature of the triple point of water. Amount of substance: mol The amount of a substance that contains Avogadro’s number NAvo = 6.0221 × 1023 of atoms or molecules. -

Applied Spectroscopy Spectroscopic Nomenclature
Applied Spectroscopy Spectroscopic Nomenclature Absorbance, A Negative logarithm to the base 10 of the transmittance: A = –log10(T). (Not used: absorbancy, extinction, or optical density). (See Note 3). Absorptance, α Ratio of the radiant power absorbed by the sample to the incident radiant power; approximately equal to (1 – T). (See Notes 2 and 3). Absorption The absorption of electromagnetic radiation when light is transmitted through a medium; hence ‘‘absorption spectrum’’ or ‘‘absorption band’’. (Not used: ‘‘absorbance mode’’ or ‘‘absorbance band’’ or ‘‘absorbance spectrum’’ unless the ordinate axis of the spectrum is Absorbance.) (See Note 3). Absorption index, k See imaginary refractive index. Absorptivity, α Internal absorbance divided by the product of sample path length, ℓ , and mass concentration, ρ , of the absorbing material. A / α = i ρℓ SI unit: m2 kg–1. Common unit: cm2 g–1; L g–1 cm–1. (Not used: absorbancy index, extinction coefficient, or specific extinction.) Attenuated total reflection, ATR A sampling technique in which the evanescent wave of a beam that has been internally reflected from the internal surface of a material of high refractive index at an angle greater than the critical angle is absorbed by a sample that is held very close to the surface. (See Note 3.) Attenuation The loss of electromagnetic radiation caused by both absorption and scattering. Beer–Lambert law Absorptivity of a substance is constant with respect to changes in path length and concentration of the absorber. Often called Beer’s law when only changes in concentration are of interest. Brewster’s angle, θB The angle of incidence at which the reflection of p-polarized radiation is zero. -

Black Body Radiation and Radiometric Parameters
Black Body Radiation and Radiometric Parameters: All materials absorb and emit radiation to some extent. A blackbody is an idealization of how materials emit and absorb radiation. It can be used as a reference for real source properties. An ideal blackbody absorbs all incident radiation and does not reflect. This is true at all wavelengths and angles of incidence. Thermodynamic principals dictates that the BB must also radiate at all ’s and angles. The basic properties of a BB can be summarized as: 1. Perfect absorber/emitter at all ’s and angles of emission/incidence. Cavity BB 2. The total radiant energy emitted is only a function of the BB temperature. 3. Emits the maximum possible radiant energy from a body at a given temperature. 4. The BB radiation field does not depend on the shape of the cavity. The radiation field must be homogeneous and isotropic. T If the radiation going from a BB of one shape to another (both at the same T) were different it would cause a cooling or heating of one or the other cavity. This would violate the 1st Law of Thermodynamics. T T A B Radiometric Parameters: 1. Solid Angle dA d r 2 where dA is the surface area of a segment of a sphere surrounding a point. r d A r is the distance from the point on the source to the sphere. The solid angle looks like a cone with a spherical cap. z r d r r sind y r sin x An element of area of a sphere 2 dA rsin d d Therefore dd sin d The full solid angle surrounding a point source is: 2 dd sind 00 2cos 0 4 Or integrating to other angles < : 21cos The unit of solid angle is steradian. -

CNT-Based Solar Thermal Coatings: Absorptance Vs. Emittance
coatings Article CNT-Based Solar Thermal Coatings: Absorptance vs. Emittance Yelena Vinetsky, Jyothi Jambu, Daniel Mandler * and Shlomo Magdassi * Institute of Chemistry, The Hebrew University of Jerusalem, Jerusalem 9190401, Israel; [email protected] (Y.V.); [email protected] (J.J.) * Correspondence: [email protected] (D.M.); [email protected] (S.M.) Received: 15 October 2020; Accepted: 13 November 2020; Published: 17 November 2020 Abstract: A novel approach for fabricating selective absorbing coatings based on carbon nanotubes (CNTs) for mid-temperature solar–thermal application is presented. The developed formulations are dispersions of CNTs in water or solvents. Being coated on stainless steel (SS) by spraying, these formulations provide good characteristics of solar absorptance. The effect of CNT concentration and the type of the binder and its ratios to the CNT were investigated. Coatings based on water dispersions give higher adsorption, but solvent-based coatings enable achieving lower emittance. Interestingly, the binder was found to be responsible for the high emittance, yet, it is essential for obtaining good adhesion to the SS substrate. The best performance of the coatings requires adjusting the concentration of the CNTs and their ratio to the binder to obtain the highest absorptance with excellent adhesion; high absorptance is obtained at high CNT concentration, while good adhesion requires a minimum ratio between the binder/CNT; however, increasing the binder concentration increases the emissivity. The best coatings have an absorptance of ca. 90% with an emittance of ca. 0.3 and excellent adhesion to stainless steel. Keywords: carbon nanotubes (CNTs); binder; dispersion; solar thermal coating; absorptance; emittance; adhesion; selectivity 1. -

Reflectometers for Absolute and Relative Reflectance
sensors Communication Reflectometers for Absolute and Relative Reflectance Measurements in the Mid-IR Region at Vacuum Jinhwa Gene 1 , Min Yong Jeon 1,2 and Sun Do Lim 3,* 1 Institute of Quantum Systems (IQS), Chungnam National University, Daejeon 34134, Korea; [email protected] (J.G.); [email protected] (M.Y.J.) 2 Department of Physics, College of Natural Sciences, Chungnam National University, Daejeon 34134, Korea 3 Division of Physical Metrology, Korea Research Institute of Standards and Science, Daejeon 34113, Korea * Correspondence: [email protected] Abstract: We demonstrated spectral reflectometers for two types of reflectances, absolute and relative, of diffusely reflecting surfaces in directional-hemispherical geometry. Both are built based on the integrating sphere method with a Fourier-transform infrared spectrometer operating in a vacuum. The third Taylor method is dedicated to the reflectometer for absolute reflectance, by which absolute spectral diffuse reflectance scales of homemade reference plates are realized. With the reflectometer for relative reflectance, we achieved spectral diffuse reflectance scales of various samples including concrete, polystyrene, and salt plates by comparing against the reference standards. We conducted ray-tracing simulations to quantify systematic uncertainties and evaluated the overall standard uncertainty to be 2.18% (k = 1) and 2.99% (k = 1) for the absolute and relative reflectance measurements, respectively. Keywords: mid-infrared; total reflectance; metrology; primary standard; 3rd Taylor method Citation: Gene, J.; Jeon, M.Y.; Lim, S.D. Reflectometers for Absolute and 1. Introduction Relative Reflectance Measurements in Spectral diffuse reflectance in the mid-infrared (MIR) region is now of great interest the Mid-IR Region at Vacuum. -

Analyzing Reflectance Data for Various Black Paints and Coatings
Analyzing Reflectance Data for Various Black Paints and Coatings Mimi Huynh US Army NVESD UNITED STATES OF AMERICA [email protected] ABSTRACT The US Army NVESD has previously measured the reflectance of a number of different levels of black paints and coatings using various laboratory and field instruments including the SOC-100 hemispherical directional reflectometer (2.0 – 25 µm) and the Perkin Elmer Lambda 1050 (0.39 – 2.5 µm). The measurements include off-the-shelf paint to custom paints and coatings. In this talk, a number of black paints and coatings will be presented along with their reflectivity data, cost per weight analysis, and potential applications. 1.0 OVERVIEW Black paints and coatings find an important role in hyperspectral imaging from the sensor side to the applications side. Black surfaces can enhance sensor performance and calibration performance. On the sensor side, black paints and coatings can be found in the optical coatings, mechanical and enclosure coating. Black paints and coating can be used inside the sensor to block or absorb stray light, preventing it from getting to the detector and affecting the imagery. Stray light can affect the signal-to-noise ratio (SNR) as well introduce unwanted photons at certain wavelengths. Black paints or coatings can also be applied to a baffle or area around the sensor in laboratory calibration with a known light source. This is to ensure that no stray light enter the measurement and calculations. In application, black paints and coatings can be applied to calibration targets from the reflectance bands (VIS- SWIR) and also in the thermal bands (MWIR-LWIR). -

Reflectance IR Spectroscopy, Khoshhesab
11 Reflectance IR Spectroscopy Zahra Monsef Khoshhesab Payame Noor University Department of Chemistry Iran 1. Introduction Infrared spectroscopy is study of the interaction of radiation with molecular vibrations which can be used for a wide range of sample types either in bulk or in microscopic amounts over a wide range of temperatures and physical states. As was discussed in the previous chapters, an infrared spectrum is commonly obtained by passing infrared radiation through a sample and determining what fraction of the incident radiation is absorbed at a particular energy (the energy at which any peak in an absorption spectrum appears corresponds to the frequency of a vibration of a part of a sample molecule). Aside from the conventional IR spectroscopy of measuring light transmitted from the sample, the reflection IR spectroscopy was developed using combination of IR spectroscopy with reflection theories. In the reflection spectroscopy techniques, the absorption properties of a sample can be extracted from the reflected light. Reflectance techniques may be used for samples that are difficult to analyze by the conventional transmittance method. In all, reflectance techniques can be divided into two categories: internal reflection and external reflection. In internal reflection method, interaction of the electromagnetic radiation on the interface between the sample and a medium with a higher refraction index is studied, while external reflectance techniques arise from the radiation reflected from the sample surface. External reflection covers two different types of reflection: specular (regular) reflection and diffuse reflection. The former usually associated with reflection from smooth, polished surfaces like mirror, and the latter associated with the reflection from rough surfaces.