The Impact of Beech Thickets on Biodiversity
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Evolution of the Brazilian Phytogeography Classification Systems: Implications for Biodiversity Conservation
Ciência e Cultura 51(5/6): 331-348, 1999. Evolution of the Brazilian phytogeography classification systems: implications for biodiversity conservation. JOLY, C.A.1; AIDAR, M.P.M.2;KLINK, C.A.3; McGRATH, D.G.4,5; MOREIRA, A. G.6; MOUTINHO, P.5; NEPSTAD, D.C.5,7; OLIVEIRA, A. A.8; POTT, A.9; RODAL, M.J.N.10 & SAMPAIO, E.V.S.B.11 1 Depto. Botânica, IB, UNICAMP, CP 6109, 13083-970, Campinas, SP (mailto:[email protected]) 2 Seção de Fisiologia e Bioquímica de Plantas, Instituto de Botânica, CP 4005, 01061-970, São Paulo, SP ([email protected])([email protected]) 3 Depto. de Ecologia, Universidade de Brasilia, C.P. 04631, 70919-970, Brasilia, DF. ([email protected]) 4 Núcleo de Altos Estudos Amazônicos, UFPa, Campus Guamá, 66075-970, Belém, PA. 5 Instituto de Pesquisa Ambiental da Amazônia, Trav. Enéias Pinheiro, 1426, Marco, 66095-100 Belém, PA. ([email protected])([email protected]) 6The Woods Hole Research Center &Instituto de Pesquisa Ambiental da Amazônia, SCLN 210, bloco C, sala 209, 70865-530, Brasilia, DF ([email protected]) 7The Woods Hole Research Center, Woods Hole, MA 02543, USA. 8Universidade de São Paulo, USP, Instituto de Biociências, Ecologia. Rua Matão 321, trav. 14.05008-900. São Paulo, SP. 9Centro de Pesquisa Agropecuária do Pantanal, EMBRAPA, R. 21 de Setembro, 1880 , 79.320-900, Corumbá, MS (mailto:[email protected]) 10Departamento de Energia Nuclear, UFPE, Av. Prof. Luís Freire 1000, 50740-540, Recife, PE ([email protected]) 11Departamento de Biologia, UFRPE, R. -

Shrub Thicket Establishment and Management
HABITAT MANAGEMENT FACT SHEET Nebraska Pheasants Forever Shrub Thicket Establishment and Management January 2015 Establishing and Managing Shrub Thickets for Wildlife Shrubby cover is an essential part of weather. To accomplish this, the shrub the habitat planning process for bobwhite thicket should be a minimum size of quail, but can also be a useful addition for 1500 ft2 (30’ x 50’ block). To ensure the prairie grouse, pheasants, deer, or even thicket is dense enough to provide non-game species such as songbirds or adequate protection a 3’ x 3’ spacing is pollinators. Because of their small acre recommended. Avoid using multiple requirement, they are a minimal impact species within a thicket. Instead plant habitat addition that can find a place on multiple thickets each with a different The average shrub thicket planting is planted in any farm or ranch. species. a 30’ x 50’ block . Each shrub is planted on 3’x3’ spacing providing adequate density for escape Species Selection Location cover once established. Native shrubs such as American Consider locations in which you plum, chokecherry, elderberry, dogwood, could imagine a natural thicket sand cherry, silver buffaloberry, establishing: drainages, low areas and skunkbush sumac, common snowberry valleys are ideal. These lower areas tend and Wood’s rose are the most common to collect additional water and can help selections. Consider planting tall, keep thickets thriving during periods of suckering species (American plum, low rainfall. Avoid hilltops and potential chokecherry, etc.) for upland bird species lekking sites for prairie grouse. like ring-necked pheasants and northern Some species require more shrubby bobwhite quail. -

A Classification of the Subtropical Transitional Thicket in the Eastern Cape, Based on Syntaxonomic and Structural Attributes
S. Afr. J. Bot., 1987, 53(5): 329 - 340 329 A classification of the subtropical transitional thicket in the eastern Cape, based on syntaxonomic and structural attributes D.A. Everard Department of Plant Sciences, Rhodes University, Grahamstown, 6140 Republic of South Africa Accepted 11 June 1987 Subtropical transitional thicket, traditionally known as valley bushveld, covers a significant proportion of the eastern Cape. This paper attempts to classify the subtropical transitional thicket into syntaxonomic and structural units and relate it to other thicket types on a continental basis. Twelve sites along a rainfall gradient were sampled for floristic and structural attributes. The floristic data were classified using TWINSPAN. Results indicate that the class subtropical transitional thicket has at least two orders of vegetation, namely kaffrarian thicket and kaffrarian succulent thicket. Two forms of thicket were recognized for both these orders viz. mesic kaffrarian thicket and xeric kaffrarian thicket for the kaffrarian thicket and mesic succulent thicket and xeric succulent thicket for the kaffrarian succulent thicket. Ordination of site data by DECORANA grouped sites according to these vegetation categories and in a sequence along axis 1 to which the rainfall gradient can be clearly related. Variation within the mesic kaffrarian thicket was however greater than between some of the other thicket types, indicating that more data are required before these forms of thicket can be formalized. Composition, endemism, diversity and the environmental controls on the distribution of the thicket types are discussed. 'n Aansienlike gedeelte van die Oos-Kaap word beslaan deur subtropiese oorgangsruigte, wat tradisioneel as valleibosveld bekend is. Hierdie studie is 'n poging om subtropiese oorgangsruigte in sintaksonomiese en strukturele eenhede te klassifiseer en dit op 'n kontinentale basis in verband met ander ruigtetipes te bring. -

PHYTOGEOGRAPHY of NAMIBIA: I.E. Geographical Distributions of Plants
PHYTOGEOGRAPHY OF NAMIBIA: i.e. geographical distributions of plants. The world flora may be mapped in different ways depending on the botanical criteria used for defining mapping units. When spatial data are used without considering the precise criteria used to define such data, results may be confusing. Two research traditions in biogeography are generally recognized, namely ecological and historical biogeography (Rosen 1988), however there are approaches that fit neither of these traditions. Ecological phytogeography is based on growth form of plants regardless of their taxonomic identity, e.g. biomes; or based on growth form and/or floristic composition, regardless of distribution ranges of taxa. Historical phytogeography is based on the total geographical range of taxa, regardless of their growth forms, e.g. the flora (the constituent plant taxa of an area, usually global); or phytogeographic region (an area with its own distinctive complement of species). It is a taxon-centred approach focussed on species, genera and/or families. The follow maps show the various ways in which the vegetation and flora of Namibia have been interpreted and mapped. Biomes Vegetation Vegetation structure structure Biomes Endemism Diversity Endemism Diversity Mendelsohn et al. 2002. Atlas of Namibia. David Phillip Publishers, Cape Town. White (1983)’s divisions in Namibia 22 Dry deciduous forest & secondary grassland 28 Mopane woodland & scrub woodland 35 Transition:woodland to Acacia deciduous bushland & wooded grassland. 36 Transition: mopane scrub woodland to Karoo-Namib shrubland 44 Kalahari Acacia wooded grassland & deciduous bushland 47 Brachystegia thicket && edaphic grassland 51 Bushy Karoo-Namib shrubland 56 Kalahari/Karoo-Namib transition 74 The Namib desert (Namib) 75 Herbabeous swamp & aquatic 76 Halophytic vegetation Kaokoveld Gariep Centres of plant diversity and endemism (Van Wyk & Smith 2000 ) The Greater Cape Floristic Region (GCFR) Is it only south of the border? Or in Namibia? (Born, J., Linder, H.P. -

Terrestrial Fauna Impact Assessment
July 2014 ENVIRONMENTAL IMPACT ASSESSMENT FOR SASOL PSA AND LPG PROJECT TERRESTRIAL FAUNA IMPACT ASSESSMENT Specialist Report 10 OOD OF MARK WOOD CONSULTANTS SSOCIADOS MOZAMBIQUE LDA PREPARED BY Author: AR Deacon Submitted to: EIA CONDUCTED BY GOLDER A WITH EIA LEADERSHIP BY MARK W SASOL Petroleum Mozambique Limitada & Sasol Petroleum Temane Limitada Report Number: 1302793 - 10712 - 20 (Eng) TERRESTRIAL FAUNA NON TECHNICAL SUMMARY Introduction Sasol Petroleum Mozambique (SPM) and Sasol Petroleum Temane (SPT) are proposing to develop the PSA Development and Liquefied Petroleum Gas (LPG) Project, situated near Inhassoro in the Inhambane Province of Mozambique. The project is an expansion of the existing Sasol Natural Gas Project in this area. Proposed new infrastructure includes 19 wells (oil and gas), associated flowlines and a new Manifold Station (8.8 ha), from which the oil flowlines will be combined into a single pipeline routed to the new Integrated PSA Liquids and LPG Plant (9.5 ha), constructed adjacent to the Central Processing Facility (CPF). This Study This study presents the findings of an assessment of the impact of the project on Terrestrial Fauna. It is one of a series of studies prepared for the Environmental Impact Assessment for the project. The study takes into account Mozambique laws and regulations, regional conventions and protocols and importantly, the Performance Standards of the International Finance Corporation, in particular Performance Standard 6, Biodiversity Conservation and Sustainable Management of Living Natural Resources, as the underpinning of the assessment and the recommendations made in the report. Methodology The survey made use of habitat availability in the different vegetation types, while the presence of observed species was used as an indicator of habitat integrity. -

From Bush Encroachment to Biome Shift: the Ecology of Thicket Pioneers
From bush encroachment to biome shift: the ecology of thicket pioneers Susi Vetter Botany Department Rhodes University Parr et al. (2012) distinguished between savanna thickening and thicket expansion Savanna Thicket encroachment Closed-canopy thicket Which spp? What is their effect on the grass layer? What are their traits? e.g. Parr et al. (2012) in Hluhluwe: “new thickets” characterized by Diospyros simii, Berchemia zeyheri Effect of woody composition and structure on herbaceous cover and composition • 3 Sites • Fine scale data (5m diameter circular plots) • Path analysis (SEM) Species associated with tall / dense woody cover Woody Woody structure composition (canopy area, (no. stems) height) Effect of particular species Effect of tall and dense on grass canopy on grasses Herbaceous basal cover and composition Data: Daisy Chiloane, Susi Vetter Eastern Cape Bhisho Thornveld; commercial cattle + game; MAP ~ 650 mm Brachylaena Canopy elliptica Vachellia area Grewia karroo occidentalis Canopy height Scutia Woody myrtina Woody 0.66 structure Maytenus composition R2 = 0.44 heterophylla -0.31 N = 235 -0.38 Karochloa Basal cover curva Herbaceous R2 = 0.40 Grass height Panicum maximum Digitaria eriantha Forb Sporobolus Themeda fimbriatus triandra Thicket pioneer spp. – what are their functional traits? Ability to colonize Ability to transform • Dispersal • Accumulation of deep canopies • Bud protection, bark thickness, growth rate • High LAI • Resprouting strategies • Adaptations to escape browse trap Bark thickness: 32 Savanna Canopy: Pioneer -

The Historical Phytogeography of Cirsium Arvense, an Invasive Species in Pennsylvania
University of Pennsylvania ScholarlyCommons Internship Program Reports Education and Visitor Experience 2018 The Historical Phytogeography of Cirsium arvense, An Invasive Species in Pennsylvania Janet K. Mansaray University of Pennsylvania Follow this and additional works at: https://repository.upenn.edu/morrisarboretum_internreports Part of the Botany Commons Recommended Citation Mansaray, Janet K., "The Historical Phytogeography of Cirsium arvense, An Invasive Species in Pennsylvania" (2018). Internship Program Reports. 6. https://repository.upenn.edu/morrisarboretum_internreports/6 An independent study project report by The Eli Kirk Price Endowed Flora of Pennsylvania Intern (2017-2018) This paper is posted at ScholarlyCommons. https://repository.upenn.edu/morrisarboretum_internreports/6 For more information, please contact [email protected]. The Historical Phytogeography of Cirsium arvense, An Invasive Species in Pennsylvania Abstract According to the Department of Conservation and Natural Resources, Cirsium arvense (Asteraceae family) is currently an invasive plant in the state of Pennsylvania. Invasive species pose a problem as they are detrimental to natural ecosystems and very costly to manage and eradicate. In this study, distribution of C. arvense in Pennsylvania was reconstructed using only herbarium records. Through detailed methodology, it was determined that there were no shifts in habit preference over time. With the data being specific ot Pennsylvania, the objective was to determine if the distribution and habitat preference would align with the current literature on what is known about C. arvense. The data seemed to support the current literature in that C. arvense appeared to be widespread and prefers dry, disturbed areas like roadsides. However, with further analysis, the data was found to reflect trends in field collecting as opposed to the distribution of the species. -

Miombo Ecoregion Vision Report
MIOMBO ECOREGION VISION REPORT Jonathan Timberlake & Emmanuel Chidumayo December 2001 (published 2011) Occasional Publications in Biodiversity No. 20 WWF - SARPO MIOMBO ECOREGION VISION REPORT 2001 (revised August 2011) by Jonathan Timberlake & Emmanuel Chidumayo Occasional Publications in Biodiversity No. 20 Biodiversity Foundation for Africa P.O. Box FM730, Famona, Bulawayo, Zimbabwe PREFACE The Miombo Ecoregion Vision Report was commissioned in 2001 by the Southern Africa Regional Programme Office of the World Wide Fund for Nature (WWF SARPO). It represented the culmination of an ecoregion reconnaissance process led by Bruce Byers (see Byers 2001a, 2001b), followed by an ecoregion-scale mapping process of taxa and areas of interest or importance for various ecological and bio-physical parameters. The report was then used as a basis for more detailed discussions during a series of national workshops held across the region in the early part of 2002. The main purpose of the reconnaissance and visioning process was to initially outline the bio-physical extent and properties of the so-called Miombo Ecoregion (in practice, a collection of smaller previously described ecoregions), to identify the main areas of potential conservation interest and to identify appropriate activities and areas for conservation action. The outline and some features of the Miombo Ecoregion (later termed the Miombo– Mopane Ecoregion by Conservation International, or the Miombo–Mopane Woodlands and Grasslands) are often mentioned (e.g. Burgess et al. 2004). However, apart from two booklets (WWF SARPO 2001, 2003), few details or justifications are publically available, although a modified outline can be found in Frost, Timberlake & Chidumayo (2002). Over the years numerous requests have been made to use and refer to the original document and maps, which had only very restricted distribution. -

Vertebrate Richness and Biogeography in the Big Thicket of Texas
Am. Midl. Nat. 164:37–43 Vertebrate Richness and Biogeography in the Big Thicket of Texas 1 1 MICHAEL H. MACROBERTS AND BARBARA R. MACROBERTS Herbarium, Museum of Life Sciences, Louisiana State University in Shreveport, Shreveport 71115 and Bog Research, 740 Columbia, Shreveport, Louisiana 71104 AND D. CRAIG RUDOLPH Wildlife Habitat and Silviculture Laboratory (maintained in cooperation with the College of Forestry, Stephen F. Austin State University), Southern Research Station, USDA Forest Service, Nacogdoches, Texas 75962 ABSTRACT.—The Big Thicket of Texas has been described as rich in species and a ‘‘crossroads:’’ a place where organisms from many different regions meet. We examine the species richness and regional affiliations of Big Thicket vertebrates. We found that the Big Thicket is neither exceptionally rich in vertebrates nor is it a crossroads for vertebrates. Its vertebrate fauna is predominantly eastern. INTRODUCTION The Big Thicket of southeastern Texas has been repeatedly described as exceptionally rich in species, diverse in plant communities and a crossroads where adjacent ecological regions meet and mingle (see Cozine, 2004; MacRoberts and MacRoberts, 2004, 2007, 2009; Callicott et al., 2006; Diggs et al., 2006 for history of this description and references). Probably the most cited example of this view is Eisner (1973: 525) when he wrote that the Big Thicket was ‘‘a region of extraordinary biological exuberance … ecologically unique not only to Texas, but to the entire North American expanse as well. Located at the crossroads between forests of the South and East and the vegetation of the West, the Thicket includes … elements from all convergent zones. It is the way which diversity of kind is combined with diversity of association that gives the area its special mark.’’ This view has been and continues to be repeated despite the lack of evidence to support it (see Gunter, 1993; Peacock, 1994; Watson, 2006 for examples of the view and references, and National Park Service, 2009a, b; UNESCO Biosphere Reserves, 2009, for current on-line examples). -
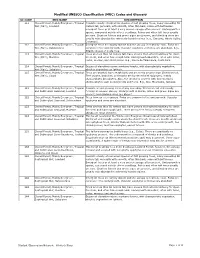
Modified UNESCO Classification (MUC) Codes and Glossary
Modified UNESCO Classification (MUC) Codes and Glossary UC CODE MUC NAME DESCRIPTION 111 Closed Forest, Mainly Evergreen, Tropical Consists usually of numerous species of fast growing trees, many exceeding 50 Wet (Rain), Lowland meters tall, generally with smooth, often thin bark, some with buttresses. Emergent trees or at least a very uneven canopy often present. Undergrowth is sparse, composed mainly of tree seedlings. Palms and other tuft trees usually are rare. Crustose lichens and green algae are present, and climbing vines are usually only abundant in extremely humid regions (e.g., Sumatra, Atrato Valley, Columbia). 112 Closed Forest, Mainly Evergreen, Tropical Emergent trees are largely absent and the canopy is relatively even. Forbs are Wet (Rain), Submontane common in the undergrowth. Vascular epiphytes and vines are abundant. E.g., Atlantic slopes of Costa Rica. 113 Closed Forest, Mainly Evergreen, Tropical Trees are less than 50 meters tall, have crowns that extend relatively far down Wet (Rain), Montane the stem, and often have rough bark. Undergrowth abundant, often with ferns, herbs, mosses, and small palms. E.g., Sierra de Talamanca, Costa Rica. 114 Closed Forest, Mainly Evergreen, Tropical Occurs at elevations above montane forests, with characteristic vegetation, Wet (Rain), Subalpine which is dependent on latitude. 115 Closed Forest, Mainly Evergreen, Tropical Trees are gnarled, have rough bark and are rarely greater than 20 meters tall. Wet (Rain), Cloud Tree crowns, branches, and trunks are burdened with epiphytes, mainly chamaephytic bryophytes. Also, the ground is covered with hygromorphic chamaephytes such as Selaginella and ferns. E.g., Blue Mountains, Jamaica. 121 Closed Forest, Mainly Evergreen, Tropical Consists of fast growing trees, many exceeding 50 meters tall and usually and Subtropical Seasonal, Lowland forming an uneven canopy. -

Presentation to Set the Stage for the Field Trips and Discussion
1 2 3 4 Introduction We wanted to have a brief presentation to set the stage for the field trips and discussion. So we’ll be talking briefly about desired conditions, current conditions, vegetation management challenges, and tools available to meet desired conditions Desired conditions paint a picture of the desired outcomes of land management. So what do we want our Ponderosa pine forests to look like? This will also help inform what to look for and think about as we are on our field trip. Will be adapted to new science/information 5 Key elements are composition, structure, processes, and function Desired Condition characteristics are often presented in ranges, not single targets, to account for variability across the landscape due to topographic characteristics, soils, microclimates, etc… We are not necessarily trying to return the forest to historic conditions, but are trying to restore natural composition, structure, processes, and functions, to create sustainable and resilient forest ecosystems. 6 Many vegetation types are departed from desired conditions. At our assessment community meetings, when we asked about conditions, trends, and risks, people overwhelmingly identified more woody vegetation. We see this especially with woody species infill in woodlands and forests and encroachment in grasslands and other gaps, openings (refer to photo). Now have vertical and lateral continuity and overall increases in fuel loading uncharacteristic of historic conditions. In some areas, vegetation structure has shifted from large to very- large size trees to a preponderance of small to medium size trees, which has also reduced the density, vigor and productivity of herbaceous understory plants in woodlands. -

INVESTING in SUSTAINABILITY Restoring Degraded Thicket, Creating Jobs, Capturing Carbon and Earning Green Credit
INVESTING IN SUSTAINABILITY Restoring degraded thicket, creating jobs, capturing carbon and earning green credit Lead Contributor: Dr Anthony Mills Contributors: Prof James Blignaut, Prof Richard Cowling, Andrew Knipe, Dr Christo Marais, Sarshen Marais, Dr Anthony Mills, Mike Powell, Dr Ayanda Sigwela, Andrew Skowno Editors: Shirley Pierce and Andrew Skowno September 2009 1 | P a g e Restoring degraded thicket, creating jobs, capturing carbon and earning green credit Lead Contributor Dr Anthony Mills Contributors Prof James Blignaut, Prof Richard Cowling, Andrew Knipe, Dr Christo Marais, Sarshen Marais, Dr Anthony Mills, Dr Shirley Pierce, Mike Powell, Dr Ayanda Sigwela Editors Dr Shirley Pierce and Andrew Skowno Principal funding of document DG Murray Trust administered via the Climate Action Partnership Project funding and support for document Working for Woodlands Programme, Department of Water Affairs (DWA) Implementation and additional funding for document Wilderness Foundation Contributors and their affiliations Prof James Blignaut, Department of Economics, University of Pretoria, Africa's Search for Sound Economic Trajectories (ASSET) Prof Richard Cowling, Botany Department, Nelson Mandela Metropolitan University, Restoration Research Group (R3G) Andrew Knipe, Gamtoos Irrigation Board (GIB) and Department of Water and Environment Affairs (DWEA) Dr Christo Marais, Working for Woodlands Programme and Working for Water Programme, Department of Water Affairs (DWA) Sarshen Marais, Climate Action Partnership (CAP) Dr Anthony Mills, Department