To View Our Latest Update
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
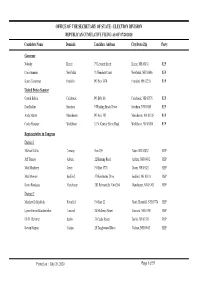
ELECTION DIVISION REPUBLICAN CUMULATIVE FILING AS of 07/20/2020 Candidate Name Domicile Candidate Address City/State/Zip Party
OFFICE OF THE SECRETARY OF STATE - ELECTION DIVISION REPUBLICAN CUMULATIVE FILING AS OF 07/20/2020 Candidate Name Domicile Candidate Address City/State/Zip Party Governor Nobody Keene 75 Leverett Street Keene, NH 03431 REP Chris Sununu Newfields 71 Hemlock Court Newfields, NH 03856 REP Karen Testerman Franklin PO Box 3874 Franklin, NH 03235 REP United States Senator Gerard Beloin Colebrook PO BOx 86 Colebrook, NH 03576 REP Don Bolduc Stratham 5 Winding Brook Drive Stratham, NH 03885 REP Andy Martin Manchester PO Box 742 Manchester, NH 03105 REP Corky Messner Wolfeboro 33 N. Kenney Shore Road Wolfeboro, NH 03894 REP Representative in Congress District 1 Michael Callis Conway Box 259 Eaton, NH 03832 REP Jeff Denaro Auburn 22 Hunting Road Auburn, NH 03032 REP Matt Mayberry Dover PO Box 1776 Dover, NH 03821 REP Matt Mowers Bedford 37 Hawthorne Drive Bedford, NH 03110 REP Kevin Rondeau Manchester 282 Belmont St., Unit 204 Manchester, NH 03103 REP District 2 Matthew D. Bjelobrk Haverhill PO Box 22 North Haverhill, NH 03774 REP Lynne Ferrari Blankenbeker Concord 26 Mulberry Street Concord, NH 03301 REP Eli D. Clemmer Berlin 35 Cedar Street Berlin, NH 03570 REP Steven Negron Nashua 28 Tanglewood Drive Nashua, NH 03062 REP Printed on : July 20, 2020 Page 1 of 51 OFFICE OF THE SECRETARY OF STATE - ELECTION DIVISION REPUBLICAN CUMULATIVE FILING AS OF 07/20/2020 Candidate Name Domicile Candidate Address City/State/Zip Party Executive Councilor District 1 Joseph D. Kenney Wakefield PO Box 201 Union, NH 03887 REP Kim Strathdee Lincoln PO Box 581 Lincoln, NH 03251 REP District 2 Jim Beard Lempster PO Box 3 Lempster, NH 03605 REP Stewart I. -

The Koch's Criminal Justice Hypocrisy in New Hampshire
CCWWTT::^^RRWW11aa^^ccWWTTaabb´´ 666AAA000===888CCC444 BBBCCC000CCC444 ???AAA>>>999444222CCC 77^^ffccWWTTAAPPSSXXRRPP[[00VVTT]]SSPP^^UU >dc^UBcPcT1XV>X[1X[[X^]PXaTb 77PPbb77ddaacc==TTff77PP\\__bbWWXXaaTT 1 Charles and David Koch pour hundreds of millions of dollars into our political system to advance their self-enriching agenda and elect their puppet candidates. At the state and national level, the Kochs use their unlimited resources to influence policy to suit their political and personal needs while hurting middle class and working families. The policies they favor include cutting taxes for corporations and the wealthy; reducing and eliminating regulations to protect workers, consumers, and the environment; privatizing and cutting both Social Security and Medicare; and cutting other programs, including Pell Grants for college. For decades, the Kochs and their network of dark money political front groups have been pushing the Koch agenda in New Hampshire — perhaps more than any other state in the country — which has benefitted billionaires like the Kochs at the expense of Granite Staters. In 2016, New Hampshire will continue to be on center stage in American politics with the First In The Nation primary, a top- tier Senate race, marquee Congressional contests, an open governor’s mansion, and a number of hot button issues in the limelight. At the same time, the Koch network has promised to spend nearly $900 million to buy elections for candidates who will do their bidding for them. The Kochs themselves admit they “expect something in return” for the millions they spend propping up their candidates, but for candidates, backing from the Kochs comes with a high price tag. -

Office of the Secretary of State - Election Division
OFFICE OF THE SECRETARY OF STATE - ELECTION DIVISION ROSTER OF HOUSE OF REPRESENTATIVES - 11/03/2020-updated 6/16/21 Candidate Name Domicile Candidate Address City/State/Zip Party State Representative BELKNAP County District 1 Tom Ploszaj Center Harbor 137 Daniel Webster Hwy Center Harbor, NH 03226 REP District 2 Glen Aldrich Gilford 343 Old Lakeshore Road, Lot 43 Gilford, NH 03249 REP Harry H. Bean Gilford 234 Saltmarsh Pond Road Gilford, NH 03249 REP Jonathan Mackie Meredith 26 Campground Road Meredith, NH 03253 REP Norm Silber Gilford 243 Mountain Drive Gilford, NH 03249 REP District 3 Mike Bordes Laconia 266 Endicott Street N., Unit 3 Laconia, NH 03246 REP Gregg Hough Laconia 169 Highland Street Laconia, NH 03246 REP Dawn M. Johnson Laconia 199 Country Club Road Laconia, NH 03246 REP Richard Littlefield Laconia 29 Merrimac St #1 Laconia, NH 03246 REP District 4 Juliet Harvey-Bolia Tilton 66 Dunlop Drive Tilton, NH 03276 REP Timothy P. Lang, Sr. Sanbornton 140 Upper Smith Road Sanbornton, NH 03269 REP District 5 Paul A. Terry Alton 915 Stockbridge Corner Road Alton, NH 03809 REP Peter R. Varney Alton PO Box 1059 Alton, NH 03809 REP District 6 Mike Sylvia Belmont 216 Farrarville Road Belmont, NH 03220 REP Page 1 of 28 OFFICE OF THE SECRETARY OF STATE - ELECTION DIVISION ROSTER OF HOUSE OF REPRESENTATIVES - 11/03/2020-updated 6/16/21 Candidate Name Domicile Candidate Address City/State/Zip Party Douglas R. Trottier Belmont 45 Meadow Lane Belmont, NH 03220 REP District 7 Barbara Comtois Barnstead PO Box 186 Center Barnstead, NH 03225 REP District 8 Raymond Howard, Jr. -

Università Degli Studi Di Padova
UNIVERSITÀ DEGLI STUDI DI PADOVA DIPARTIMENTO DI FILOSOFIA, SOCIOLOGIA, PEDAGOGIA E PSICOLOGIA APPLICATA CORSO DI LAUREA IN COMUNICAZIONE "I LINGUAGGI DI DONALD J. TRUMP E BARACK OBAMA E LORO DIFFUSIONE GIORNALISTICA. UN CONFRONTO" Relatore: Ch.mo Prof. Raffaele Fiengo Laureando: Massimiliano Pappalardo Matricola n. 1070022 ANNO ACCADEMICO 2015- 2016 INDICE INTRODUZIONE ......................................................................................................... 1 CAPITOLO 1 – BARACK HUSSEIN OBAMA .......................................................... 5 CAPITOLO 1.1 – La Convention nazionale democratica del 2004 .......................... 5 CAPITOLO 1.2 – Il discorso post-vittoria a Chicago ............................................. 12 CAPITOLO 1.3 – Il discorso all'Università del Cairo ............................................. 21 CAPITOLO 2 – DONALD J. TRUMP ....................................................................... 33 CAPITOLO 2.1 – Il discorso di candidatura ........................................................... 33 CAPITOLO 2.2 – La vittoria in New Hampshire .................................................... 43 CAPITOLO 2.3 – Trump e il Papa .......................................................................... 50 CAPITOLO 2.4 – Il giuramento di Orlando ............................................................ 55 CAPITOLO 2.5 – La tortura .................................................................................... 59 CAPITOLO 2.6 – Il secondo emendamento ........................................................... -

Hazard Mitigation Plan 2010
TOWN OF LONDONDERRY, NEW HAMPSHIRE Town of Londonderry, New Hampshire, Town Offices HAZARD MITIGATION PLAN 2010 ii TOWN OF LONDONDERRY NEW HAMPSHIRE HAZARD MITIGATION PLAN NOVEMBER 30, 2010 Prepared by the Southern New Hampshire Planning Commission The preparation of this document has been financed in part by a grant from the State of New Hampshire Department of Safety, Homeland Security and Emergency Management. Acknowledgements Appreciation is extended to the following people for contributing their time and effort to complete the Londonderry Hazard Mitigation Plan: 2010 Londonderry Hazard Mitigation Committee Members Kevin MacCaffrie - Emergency Management Director, Fire Chief, Chair Richard G. Canuel - Senior Building Inspector/ Zoning Officer, Community Development Department Sharon Carson - Resident of the Town of Londonderry Tim Jones - Lieutenant, Londonderry Police Department Tim Thompson - Town Planner, Londonderry Community Development Department John R. Trottier - Assistant Director of Public Works and Engineering, Department of Public Works Jodie Levandowski - Intern, Londonderry Community Development Department John R. Gilcreast - Assistant Building Inspector, Community Development Department Thanks also to: • The New Hampshire Department of Safety, Division of Fire Safety and Emergency Management, Homeland Security and Emergency Management (NHHSEM), which developed the New Hampshire Natural Hazards Mitigation Plan; • The Southwest Region Planning Commission, which developed Hazard Mitigation Planning for New Hampshire Communities; and • The Bedford, Derry, Goffstown, Hooksett, Manchester and New Boston Hazard Mitigation Committees and their respective Hazard Mitigation Plans. All the above publications served as models for this plan. "We will of course be there to help after disaster strikes, but as you all know, there’s no substitute for mitigation before it does... -

Allegheny County Sportsmen's League Legislative Committee Report
Allegheny County Sportsmen’s League Legislative Committee Report February 2011 Issue 196 ALLEGHENY COUNTY SPORTSMEN LEAGUE ON THE INTERNET http://www.acslpa.org Contacts : Legislative Committee Chairman , Kim Stolfer (412.221.3346) - [email protected] Legislative Committee Vice-Chairman, Mike Christeson - [email protected] Founding Fathers: "Men must be ready , they must pride themselves and be happy to sacrifice their private pleasures, passions and interests, nay, their private friendships and dearest connections, when they stand in competition with the rights of society." -- John Adams , letter to Mercy Warren, 1776 Gun Owners Targeted ‘Again’ by Media That website was set up by "a group of (ATF) employees who formed a non-profit organization dedicated to returning Big border region gun bust raises questions integrity, accountability and decency to the management of the By Dave Workman, Senior Editor (agency)," according to one of Codrea's Examiner columns. Federal indictments against more than 30 Arizona residents in January's arrests tended to reinforce contentions by the connection with alleged gun running to Mexico have some Obama administration that thousands of US-origin firearms are Internet gun rights activists raising questions. fueling the drug cartel wars in Mexico. The Justice Department announced charges in late January In one of the court documents, a 43-page indictment naming against 34 people who allegedly purchased an estimated 700 20 of the defendants, one gun shop was prominently identified as firearms in Arizona, including semiautomatic rifles and pistols, the source of scores of firearms purchased by various suspects and some .50-caliber long-range rifles. between March and July 2010. -

House Journal No. 1
HOUSE RECORD First Year of the 166th General Court Calendar and Journal of the 2019 Session State of New Hampshire Web Site Address: www.gencourt.state.nh.us Vol. 41 Concord, N.H. Wednesday, December 5, 2018 No. 1X HOUSE JOURNAL NO. 1 Wednesday, December 5, 2018 On the first Wednesday in December in the year of our Lord, two thousand eighteen, it being the day designated by the Constitution for assembling for organizational purposes, the one hundred and sixty-sixth General Court of the State of New Hampshire convened at the Capitol in the City of Concord. The Representatives-elect were called to order by Paul C. Smith, Clerk of the House for the preceding session. Prayer was offered by former House Chaplain, Reverend Roger Boucher of Gilmanton Iron Works. Dear Lord, we acknowledge You on this Organization Day as the wisdom we need and from which we draw the strength to set the plan in motion for the coming year. Guide and unite us so that we may move forward. Be the light of our minds and hearts as we discern what is Your will for the citizens of this great state. In our hearts we wish, on this national day of mourning for our 41st President of the United States, to attend the proper rites which honor the steady hand and blessings of his governing years. And so in our hearts we pray that You surround him and his family with Your love which is the final completion of everything that is meaningful. Bless those in this body taking office and our Governor with good counsel and with the virtue of holding sacred the common good of our communities. -

OFFICE of the SECRETARY of STATE - ELECTION DIVISION DEMOCRATIC CUMULATIVE FILING AS of 06/10/2016 Candidate Name Domicile Candidate Address City/State/Zip Party
OFFICE OF THE SECRETARY OF STATE - ELECTION DIVISION DEMOCRATIC CUMULATIVE FILING AS OF 06/10/2016 Candidate Name Domicile Candidate Address City/State/Zip Party Governor Mark Connolly New Castle PO Box 3450 Manchester, NH 03105 DEM Derek Dextraze Dover 60 Silver Street Dover, NH 03820 DEM Ian Freeman Keene 63 Emerald Street #610 Keene, NH 03431 DEM Steve Marchand Portsmouth 310 FW Hartford Drive Portsmouth, NH 03801 DEM Colin Van Ostern Concord P O Box 3931 Manchester, NH 03105 DEM United States Senator Maggie Hassan Newfields 3 Scanlon Way Newfields, NH 03856 DEM Representative in Congress District 1 Carol Shea-Porter Rochester PO Box 453 Rochester, NH 03866 DEM District 2 Ann McLane Kuster Hopkinton 331 Gould Hill Road Hopkinton, NH 03229 DEM Executive Councilor District 1 Michael J. Cryans Hanover PO Box 999 Hanover, NH 03755 DEM District 2 Shawn Mickelonis Rochester 56 Woodland Green Rochester, NH 03868 DEM John D. Shea Nelson 8 McIntire Road Nelson, NH 03457 DEM Andru Volinsky Concord 488 Shaker Road Concord, NH 03301 DEM District 3 Joshua Bourdon Derry 11 Village Brook Lane Derry, NH 03038 DEM Beth Roth Salem 103 Corinthian Drive Salem, NH 03079 DEM Printed on : June 10, 2016 Page 1 of 28 OFFICE OF THE SECRETARY OF STATE - ELECTION DIVISION DEMOCRATIC CUMULATIVE FILING AS OF 06/10/2016 Candidate Name Domicile Candidate Address City/State/Zip Party District 4 Chris Pappas Manchester 629 Kearney Circle Manchester, NH 03104 DEM District 5 Dan Weeks Nashua 7 Shattuck Street Nashua, NH 03064 DEM State Senator District 1 Jeff Woodburn Whitefield 30 King Square Whitefield, NH 03598 DEM District 2 Charlie Chandler Warren 400 Swain Hill Road Warren, NH 03274 DEM District 3 John R. -

Londonderry Times 10/29/2020
HOMETOWN NEWS DELIVERED TO EVERY HOME IN TOWN FREE October 29, 2020 N Volume 21 – Issue 44 A FREE Weekly Publication Planning Board Discusses Change to Elderly Housing KELSEY DERHAK the ordinance coming who is in favor of elimi- LONDONDERRY TIMES ————–––––————–N before the Planning Board nating the elderly hous- he Londonderry is due to the unintended ing ordinance. Verani in Planning Board met effects of the ordinance, agreement with the fact Ton Wednesday, Oct. which is mainly the afford- that ordinance did not 14, and discussed elimi- ability aspect of said hous- benefit the town as they nating the elderly hous- ing. Due to the incentives had hoped is also in ing ordinance. for developers to create favor of getting more The ordinance put in such housing, the prices creative to supply af- place a number of years for such housing have sur- fordable housing for the back, gave developers passed ordinary housing employees of the grow- incentives to build 55 prices. The changes to ing industrial communi- and older housing. The the ordinance would take ty in town. Verani’s con- town put in place the away the incentive of a cern is that the encour- ordinance as a means to density bonus and units agement of industrial help relieve the pressure per acre. This would give growth without supply- of school age children the developer a standard ing enough housing will and give the 55 and older 1 acre with 150 ft. of negatively impact Lon- community an affordable frontage. The developer donderry. “It is hypo- option for housing. -

Office of the Secretary of State - Election Division
OFFICE OF THE SECRETARY OF STATE - ELECTION DIVISION STATE REPRESENTATIVE ELECTION WINNERS – 2019 - 2020 Candidate Name Domicile Candidate Address City/State/Zip Party State Representative BELKNAP County District 1 Harry Viens Center Harbor 206 Coe Hill Road, PO Box 127 Center Harbor, NH 03226 REP District 2 Glen Aldrich Gilford 343 Old Lake Shore Road Gilford, NH 03249 REP Harry H. Bean Gilford 234 Saltmarsh Pond Road Gilford, NH 03249 REP Deanna Jurius Meredith 49 Stoney Brook Road Meredith, NH 03253 REP Jonathan D. Mackie Meredith 20 Campground Road Meredith, NH 03253 REP District 3 David Huot Laconia 19 Wildwood Road Laconia, NH 03246 DEM Richard B. Beaudoin Laconia PO Box 6373 Lakeport, NH 03246 REP Peter J. Spanos Laconia PO Box 102 Winnisquam, NH 03289 REP Frank Tilton Laconia 56 Orchard Street Laconia, NH 03246 REP District 4 Dennis H. Fields Sanbornton 429 Lower Bay Road Sanbornton, NH 03269 REP Timothy Lang, Sr. Sanbornton 140 Upper Smith Road Sanbornton, NH 03269 REP District 5 George Feeney Alton PO Box 221 Alton Bay, NH 03810 REP Peter Varney Alton PO Box 1059 Alton, NH 03809 REP District 6 John R. Plumer Belmont 34 Bean Hill Road Belmont, NH 03220 REP OFFICE OF THE SECRETARY OF STATE - ELECTION DIVISION STATE REPRESENTATIVE ELECTION WINNERS – 2019 - 2020 Candidate Name Domicile Candidate Address City/State/Zip Party Mike Sylvia Belmont 216 Farrarville Road Belmont, NH 03220 REP District 7 Barbara Comtois Barnstead PO Box 186 Center Barnstead, NH 03225 REP District 8 Raymond Howard, Jr. Alton 311 Stockbridge Corner Alton, NH 03809 REP District 9 Charlie St. -

Congressional Record United States Th of America PROCEEDINGS and DEBATES of the 117 CONGRESS, FIRST SESSION
E PL UR UM IB N U U S Congressional Record United States th of America PROCEEDINGS AND DEBATES OF THE 117 CONGRESS, FIRST SESSION Vol. 167 WASHINGTON, WEDNESDAY, SEPTEMBER 29, 2021 No. 170 House of Representatives The House met at 10 a.m. and was Roberta and I are praying for Olivia leadership, and I thank his entire staff called to order by the Speaker pro tem- King’s children, her family, and her for their heroism. Special commenda- pore (Ms. WILD). friends at this difficult time. tion also goes to Collierville Fire Chief f As Collierville Alderman Maureen Buddy Billings and his men and women Fraser said: ‘‘Everybody needs to be for their swift action that prevented DESIGNATION OF SPEAKER PRO more like Olivia King. She was very the additional loss of life. TEMPORE kind, very generous, very Christian, Further, I want to recognize all the The SPEAKER pro tempore laid be- and a rule-follower.’’ first responders, the doctors, the sur- fore the House the following commu- Our hearts go out to all the victims, geons, the nurses, and their staff for nication from the Speaker: their loved ones, and everyone in the delivering the necessary and imme- WASHINGTON, DC, entire community who has been im- diate care to these victims. September 29, 2021. pacted. Lastly, I would like to extend my I hereby appoint the Honorable SUSAN I want to quote Collierville Police sincere appreciation to Collierville WILD to act as Speaker pro tempore on this Chief Dale Lane, who said that Mayor Stan Joyner, Town Adminis- day. -

List of Donald Trump 2020 Presidential Campaign Endorsements
List of Donald Trump 2020 presidential campaign endorsements This is a list of notable individuals and organizations who voiced their endorsement for the office of the president of List of Donald Trump 2020 Donald Trump as the Republican Party's presidential presidential campaign candidate for the 2020 United States presidential election. endorsements Contents Current federal executive officials Former federal executive officials Campaign 2020 United States Vice Presidents presidential election Cabinet-level officials Candidate Donald Trump U.S. Ambassadors President of the United White House officials States U.S. Senators (2017–present) Current Mike Pence Former Vice President of the United States U.S. Representatives (2017–present) Current Governor of Indiana Former (2013–2017) State and territorial executive officials U.S. Representative from Governors Indiana Current (2001–2013) Former Affiliation Republican Party Lieutenant Governors Headquarters Trump Tower, Current Manhattan, New York City, Former New York (main base) Attorneys General Receipts US$358,199,769.41[1] Current (December 31, 2019) Former Website Other statewide elected officials Current www.donaldjtrump.com (https://www.donaldj Former trump.com) State and territorial legislators State and territorial senators Current Former State and territorial representatives Current Former Municipal and local officials Mayors Current Former Local officials Current Former International politicians Heads of State and Government Current Deputy Heads of State and Government Current Former