(12) Patent Application Publication (10) Pub. No.: US 2009/0068255 A1 Yu Et Al
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

Jason Dandruff Relief Treatment
JASON DANDRUFF RELIEF TREATMENT - sulfur, salicylic acid shampoo The Hain Celestial Group, Inc Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies. ---------- Drug Facts Sulfur 2.0% Salicylic Acid 2.0% Sulfur 2.0% Controls Dandruff Salicylic Acid 2.0% Controls Seborrheic Dermatitis Controls recurrence of flaking, scaling and itching associated with dandruff Helps prevent seborrheic dermatitis For external use only. Avoid contact with eyes.Rinse eyes throughly with water in case contact occurs.Discontinue use and consult your physician if irritation develops. Keep out of reach of children. If swallowed get medical help or contact Poison Center right away. For best results, use at least three times each week. Wet hair and lather,massage into scalp. Rinse and repeat if desired. Store between 40 to 100 degrees F (4 to 38 degrees C). Aqua (Water), Sodium Cocoyl Isothionate, Disodium Cocoamphodiacetate, Stearic Acid,Potassium Cocoyl Glutamate, Glycerin, Sodium Lauroyl Sarcosinate, Cetyl Alcohol, Olea Europaea (Olive) Fruit Oil (1), Pogostemon Cablin (Patchouli) Oil, Rosmarinus Officinalis (Rosemary) Leaf Oil, Simmondsia Chinensis (Jojoba) Seed Oil (1), Camphor, Dimethyl Sulfone (2), Menthol, Methyl Salicylate, Potassium Hydroxide, Sodium PCA, Xanthan Gum, Benzyl Alcohol, Capryloyl Glycine, Undecylenoyl Glycine, Amyl Cinnamal, Benzyl Benzoate, Hexyl Cinnamal, Hydroxycitronellal, Linalool, Limonene, -

Transient Receptor Potential Channels and Metabolism
Molecules and Cells Minireview Transient Receptor Potential Channels and Metabolism Subash Dhakal and Youngseok Lee* Department of Bio and Fermentation Convergence Technology, Kookmin University, BK21 PLUS Project, Seoul 02707, Korea *Correspondence: [email protected] https://doi.org/10.14348/molcells.2019.0007 www.molcells.org Transient receptor potential (TRP) channels are nonselective Montell, 2007). These cationic channels were first charac- cationic channels, conserved among flies to humans. Most terized in the vinegar fly, Drosophila melanogaster. While TRP channels have well known functions in chemosensation, a visual mechanism using forward genetic screening was thermosensation, and mechanosensation. In addition to being studied, a mutant fly showed a transient response to being sensing environmental changes, many TRP channels constant light instead of the continuous electroretinogram are also internal sensors that help maintain homeostasis. response recorded in the wild type (Cosens and Manning, Recent improvements to analytical methods for genomics 1969). Therefore, the mutant was named as transient recep- and metabolomics allow us to investigate these channels tor potential (trp). In the beginning, researchers had spent in both mutant animals and humans. In this review, we two decades discovering the trp locus with the germ-line discuss three aspects of TRP channels, which are their role transformation of the genomic region (Montell and Rubin, in metabolism, their functional characteristics, and their 1989). Using a detailed structural permeation property anal- role in metabolic syndrome. First, we introduce each TRP ysis in light-induced current, the TRP channel was confirmed channel superfamily and their particular roles in metabolism. as a six transmembrane domain protein, bearing a structural Second, we provide evidence for which metabolites TRP resemblance to a calcium-permeable cation channel (Mon- channels affect, such as lipids or glucose. -

PC22 Doc. 22.1 Annex (In English Only / Únicamente En Inglés / Seulement En Anglais)
Original language: English PC22 Doc. 22.1 Annex (in English only / únicamente en inglés / seulement en anglais) Quick scan of Orchidaceae species in European commerce as components of cosmetic, food and medicinal products Prepared by Josef A. Brinckmann Sebastopol, California, 95472 USA Commissioned by Federal Food Safety and Veterinary Office FSVO CITES Management Authorithy of Switzerland and Lichtenstein 2014 PC22 Doc 22.1 – p. 1 Contents Abbreviations and Acronyms ........................................................................................................................ 7 Executive Summary ...................................................................................................................................... 8 Information about the Databases Used ...................................................................................................... 11 1. Anoectochilus formosanus .................................................................................................................. 13 1.1. Countries of origin ................................................................................................................. 13 1.2. Commercially traded forms ................................................................................................... 13 1.2.1. Anoectochilus Formosanus Cell Culture Extract (CosIng) ............................................ 13 1.2.2. Anoectochilus Formosanus Extract (CosIng) ................................................................ 13 1.3. Selected finished -

(12) Patent Application Publication (10) Pub. No.: US 2008/0299054 A1 Chandar Et Al
US 20080299054A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2008/0299054 A1 Chandar et al. (43) Pub. Date: Dec. 4, 2008 (54) PERSONAL CARE COMPOSITIONS WITH Publication Classification ENHANCED FRAGRANCE DELVERY (51) Int. Cl. (75) Inventors: Prem Chandar, Closter, NJ (US); A6IR 8/34 (2006.01) Lin Yang, Woodbridge, CT (US) A6II 3/14 (2006.01) A6II 3/17 (2006.01) Correspondence Address: UNILEVER PATENT GROUP (52) U.S. Cl. .............. 424/54; 424/59: 424/65; 424/70.1; 800 SYLVANAVENUE, AG West S. Wing 514/588 ENGLEWOOD CLIFFS, NJ 07632-3100 (US) (57) ABSTRACT (73) Assignee: CONOPCO, INC., d/b/a UNILEVER, Englewood Cliffs, NJ A personal care product is provided which includes a fra (US) grance, a Substituted urea and a quaternary ammonium salt. The Substituted urea and quaternary ammonium salt operate (21) Appl. No.: 11/755,009 together as a scent boosting system to enhance Volatilization offragrance components upon the personal care composition (22) Filed: May 30, 2007 being first applied to human skin or hair. US 2008/0299054 A1 Dec. 4, 2008 PERSONAL CARE COMPOSITIONS WITH structure AB, wherein A is a cationic charged compo ENHANCED FRAGRANCE DELVERY nent of the salt AB, B is an anionic charged component of the salt AB, and BACKGROUND OF THE INVENTION 0013 A has a single quaternized nitrogen atom, at least two hydroxy groups and a molecular weight no 0001 1. Field of the Invention higher than about 250. 0002 The invention concerns personal care compositions which upon application to a human body Surface quickly release fragrance components thereby improving aesthetics DETAILED DESCRIPTION OF THE INVENTION of these compositions. -

Opinion of the Sccnfp Concerning an Initial List of Perfumery Materials Which Must Not Form Part of Cosmetic Products Except
SCCNFP/0392/00, final OPINION OF THE SCIENTIFIC COMMITTEE ON COSMETIC PRODUCTS AND NON-FOOD PRODUCTS INTENDED FOR CONSUMERS CONCERNING AN INITIAL LIST OF PERFUMERY MATERIALS WHICH MUST NOT FORM PART OF COSMETIC PRODUCTS EXCEPT SUBJECT TO THE RESTRICTIONS AND CONDITIONS LAID DOWN adopted by the SCCNFP during the 18th Plenary meeting of 25 September 2001 1. Terms of Reference SCCNFP/0392/00, final Opinion concerning a review on the safety of perfumery materials ____________________________________________________________________________________________ In recent years there has been concern on the safety of fragrance (perfumery) materials. Dermatologists have highlighted the frequency of allergic contact dermatitis from perfumes. Under current legislation, fragrance materials do not fall under all the requirements of Directive 76/768/EEC on cosmetic products. The 6th Amendment (93/35/EEC) provides for the labelling of ingredients on cosmetic products. However, it is not a requirement to label fragrance constituents on the packaging of cosmetic products, current legislation requires only the word parfum. In response to growing concern over this issue, the Commission was asked for positive actions with respect to legislative measures on fragrance materials. 2. Mandate The SCCNFP has been asked to respond to the following questions : 1. Does the SCCNFP agree to the inclusion of all IFRA restricted materials in the Annex III (List of substances which cosmetic products must not contain except subject to restrictions and conditions laid down)? Are the permitted levels recommended by IFRA suitable for use in the Cosmetics Directive 76/768/EEC ? 2. Does the SCCNFP agree that all materials that IFRA recommend should not be used as fragrance compounds are included in Annex II (List of substances which must not form part of the composition of cosmetic products)? 3. -

Kaffir Lime) Leaves Oil
ACTIVATING EFFECT OF AROMATHERAPY INHALATION WITH Citrus hystrix (KAFFIR LIME) LEAVES OIL ALISA STEPHANIE RASION 11". sfr«.. ¢ý'si: .... : lJ1ii Liý/: r. `t , ýi THESIS SUBMITTED IN FULFILLMENT FOR THE DEGREE OF MASTER OF SCIENCE FACULTY OF SCIENCE AND NATURAL RESOURCES UNIVERSITY OF MALAYSIA SABAH 2018 UNIVERSITI MALAYSIA SABAH BORANGPENGESAHAN STATUS TESIS JUDUL: ACTIVATINGEFFECT OF AROMATHERAPYINHALATION WITH Citrus hystrix (KAFFIR LIME) LEAVESOIL IJAZAH: SARJANA SAINS (KIMIA INDUSTRI) Saya ALISA STEPHANIE RASION, sesi 2016-2018, mengaku membenarkan tesis Sarjana ini disimpan di Perpustakaan Universiti Malaysia Sabah dengan syarat-syarat kegunaan seperti berikut: - " 1. Tesis ini adalah hak milik Universiti Malaysia Sabah 2. Perpustakaan Universiti Malaysia Sabah dibenarkan membuat salinan untuk tujuan pengajian sahaja. 3. Perpustakaan dibenarkan membuat salinan tesis ini sebagai bahan pertukaran antara institusi pengajian tinggi. 4. Sila tandakan (/): üýtºi6r: iýi:: f 11 Hi1ýc11 Uia VIYL, 'ýi ý SULIT (Mengandungi maklumat yang berdarjah keselamatan atau kepentingan Malaysia seperti yang termaktub di dalam AKTA RAHSIA 1972) TERHAD (Mengandungi maklumat TERHADyang telah ditentukan oleh organisasi/badan di mana penyelidikan dijalankan x TIDAK TERHAD DisahkanOleh, NURULAINBINTI ISMAIL PUSTAKAWAIWANAN ABAH ALISA STEPHANIERASION (TandatanganPustakawan) MS1521001T Tarikh :V October 2018 (Prof. Madya. Dr. How Siew Eng) Penyelia DECLARATION I declarethat this thesis and the work presentedin it are my own and has been generatedby me as the result of my own original research. 27t'' September2018 Alisa Sianie Rasion MS1521001T 11 CERTIFICATION NAME ALISA STEPHANIE RASION MATRICNO. MS1521001T TITLE ACTIVATING EFFECT OF AROMATHERAPY INHALATION WITH Citrus hystrix (KAFFIR LIME) LEAVES OIL DEGREE : MASTER OF SCIENCE (INDUSTRIAL CHEMISTRY) VIVA DATE 9THAUGUST 2018 CERTIFIED BY; SIGNATURE SUPERVISOR PROF. MADYA.DR. -
![PF155: Fragrance Mix [A]](https://docslib.b-cdn.net/cover/0767/pf155-fragrance-mix-a-1240767.webp)
PF155: Fragrance Mix [A]
the art and science of smart patch testing® PF155: Fragrance Mix [A] Patient information What are some products that may contain fragrance mix [A]? Your patch test result indicates that you have a contact allergy to fragrance Personal Care & Hygiene Products mix [A]. This contact allergy may cause your skin to react when it is exposed to • Baby Products this substance although it may take several days for the symptoms to appear. • Bath Oils Typical symptoms include redness, swelling, itching and fluid-filled blisters. • Cosmetics: – Aftershaves Where is fragrance mix [A] found? – Colognes Fragrance mix [A] contains eight fragrances: Geraniol, Cinnamaldehyde, – Perfumes Hydroxycitronellal, Cinnamyl alcohol, alpha-Amylcinnamaldehyde, Isoeugenol, – Soaps Eugenol, and Sandalwood oil. Fragrances can be found in most products. – Tonics They are used to add flavor or scent to a product or may mask a product’s • Hair Care unpleasant smell. They may come from natural (animals or plants) or • Lotions/Creams synthetic sources. Dental Products • Dental cements How can you avoid contact with fragrance mix [A]? • Impression materials Avoid products that list any of the following names in the ingredients: • Mouthwash • alpha-amylcinnamaldehyde • Periodontal packings • Amyl cinnamal • Toothache drops or gels • Amylcinnamaldehyde • Toothpastes • Cinnamal • Cinnamaldehyde Inside the Home • Cinnamic alcohol • Household Cleaners • Cinnamic aldehyde • Laundry Products: • Cinnamyl alcohol – Detergents – Dryer sheets • Eugenol – Fabric softener • Evernia prunastri • Geraniol Medicaments • Hydroxycitronellal • Analgesics • Isoeugenol • Antiseptics • Sandalwood oil • Topical medication • Santalum album oil Foods (Flavor and Fragrance) • Breath mints • Candy • Cassia oils (cinnamon flavor) • Ice cream • Pastries • Soft drinks *For additional information about products that might contain one of the above listed fragrances, go to the Household Product Database online (householdproducts.nlm.nih.gov) at the United States National Library of Medicine. -

(12) Patent Application Publication (10) Pub. No.: US 2014/0051633 A1 Harding Et Al
US 20140051633A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2014/0051633 A1 Harding et al. (43) Pub. Date: Feb. 20, 2014 (54) HEPATOCYTE GROWTH FACTOR MIMICS (60) Provisional application No. 61/706,567, filed on Sep. AS THERAPEUTICAGENTS 27, 2012, provisional application No. 61/706,437, filed on Sep. 27, 2012. (71) Applicant: Washington State University, Pullman, WA (US) Publication Classification (72) Inventors: Joseph W. Harding, Pullman, WA (US); John W. Wright, Pullman, WA (US); (51) Int. C. Caroline C. Benoist, Nashville, TN C07K5/065 (2006.01) (US); Leen H. Kawas, Pullman, WA (52) U.S. C. (US); Gary A. Wayman, Pullman, WA CPC .................................. C07K5/06078 (2013.01) (US) USPC ........................................................... S14/95 (73) Assignee: Washington State University, Pullman, WA (US) (57) ABSTRACT (21) Appl. No.: 14/038,973 (22) Filed: Sep. 27, 2013 Small molecule, peptidic hepatocyte growth factors mimics, which act as both mimetics and antagonists, have been gen Related U.S. Application Data erated. These molecules have been shown or predicted to have (63) Continuation-in-part of application No. PCT/US2012/ therapeutic potential for numerous pathologies including 031815, filed on Apr. 2, 2012. dementia (e.g. Alzheimer's) and Parkinson's disease. Patent Application Publication Feb. 20, 2014 Sheet 1 of 40 US 2014/0051633 A1 a --- --- - - -s ------- CS -8- Scopolarine s -k- Oii exa-ow " -: N. Sks s i. Y s re. Dihexa-high as: ^ N.-- st-scs:- -------------------------------------------------------------------------------------------. Acquisition (days) Figure 1A -8-CSF -- -8- Scopoanine i. --- - -k- Dihexa-ow -- hexa-high reta-or 2 3 4 5 6 7 8 Acquisition (days) Figure 1B -8- vici-treated -- hexa Acquisition (days) Figure 1C Patent Application Publication Feb. -

CDI Allergen Info Sheets Fragrance
FRAGRANCE MIX Your patch test result indicates that you have a contact allergy to fragrance mix. This contact allergy may cause your skin to react when it is exposed to this substance although it may take several days for the symptoms to appear. Typical symptoms include redness, swelling, itching and fluid-filled blisters. Where is fragrance mix found? Fragrance mix contains the following eight substances: Geraniol, Cinnamaldehyde, Hydroxycitronellal, Cinnamyl alcohol, a-Amylcinnamaldehyde, Isoeugenol, Eugenol, and Oak moss. Fragrances can be found in most products. They are used to add flavor or scent to a product or may mask a product’s unpleasant smell. They may come from natural (animals or plants) or synthetic sources. How can you avoid contact with fragrance mix? Avoid products that list any of the following names in the ingredients: • Geraniol • Hydroxycitronellal • cinnamic alcohol • CASRN: 122-40-7 • geraniol alcohol • citronellal hydrate • CASRN: 104-54-1 • Oak moss • CASRN: 106-24-1 • CASRN: 107-75-5 • Isoeugenol • oakmoss oil or extract • Cinnamaldehyde • Eugenol • CASRN: 97-54-1 • Evernia Prunastri Lichen • Cinnamal • CASRN 97-53-0 • Amylcinnamaldehyde Extract • CASRN: 104-55-2 • Cinnamyl alcohol • amyl cinnamal • CASRN: 9000-50-4 What are some products that may contain fragrance mix? Baby products Laundry Products: • Detergents Bath Oils • Dryer Sheets Cosmetics: • Fabric Softener • Aftershaves Lotions/Creams • Colognes • Perfumes Medicaments: • Soaps • Analgesics • Tonics • Antiseptics Cutting fluids: Skin Care ® • LPS -
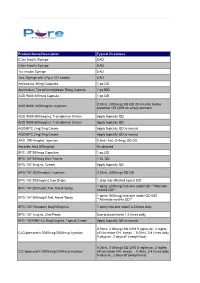
Pure Pricing List
Product Name/Description Typical Directions 0.3cc Insulin Syringe UAD 0.5cc Insulin Syringe UAD 1cc Insulin Syringe UAD 1mL Syringe with 27g x 1/2" needle UAD Amlexanox 40mg Capsules 1 po QD Ammonium Tetrathiomolybdate 50mg Capsule 1 po BID AOD 9604 600mcg Capsule 1 po QD 0.25mL (300mcg) SQ QD 30 minutes before AOD 9604 1200mcg/mL Injection breakfast OR QHS on empty stomach AOD 9604 600mcg/mL Transdermal Cream Apply topically QD AOD 9604 600mcg/mL Transdermal Cream Apply topically QD AOD/BPC 2mg/2mg Cream Apply topically QD to wound AOD/BPC 2mg/2mg Cream Apply topically QD to wound ARA 290 4mg/mL Injection 0.5mL-1mL (2-4mg) SQ QD Ascorbic Acid 500mg/mL As directed BPC-157 500mcg Capsules 1 po QD BPC-157 500mcg Mini-Troche 1 SL QD BPC-157 2mg/mL Cream Apply topically QD BPC-157 2000mcg/mL Injection 0.25mL (500mcg) SQ QD BPC-157 200mcg/mL Eye Drops 1 drop into affected eye(s) BID 1 spray (200mcg) into one nostril QD **Alternate BPC-157 200mcg/0.1mL Nasal Spray nostrils QD** 1 spray (500mcg) into one nostril QD-BID BPC-157 500mcg/0.1mL Nasal Spray **Alternate nostrils QD** BPC-157/ Synapsin 5mg/50mg/mL 1 spray into one nostril 2-3 times daily BPC-157 1mg/mL Oral Paste Use to brush teeth 1-2 times daily BPC-157/GHK-Cu 2mg/2mg/mL Topical Cream Apply topically QD to wound 0.05mL (100mcg) SQ QHS 5 nights on, 2 nights CJC/Ipamorelin 2000mcg/2000mcg Injection off (Increase GH, sleep)… 0.05mL 3-4 times daily 5 days on, 2 days off (weight loss) 0.05mL (100mcg) SQ QHS 5 nights on, 2 nights CJC/Ipamorelin 2000mcg/2000mcg Injection off (Increase GH, -

Counteracting EMF Toxicity with Peptides (Forget Global Warming Emfs Are Killing Us)
Counteracting EMF Toxicity with Peptides (Forget Global Warming EMFs are Killing Us) Dr. Kent Holtorf, MD Medical Director/CEO, Holtorf Medical Group (HoltorfMed.com) Medical Director/CEO, National Academy of Hypothyroidism (NAHypothyroidism.org) Medical Director, Integrative Peptides (IntegrativePeptides.com) Disclosure Statement Owner/CEO - Holtorf Medical Group HoltorfMed.com A multi-specialty medical group specializing in the treatment of complex endocrine dysfunction, CFS/fibromyalgia, chronic infectious diseases, immune dysfunction, neurodegenerative disease, and other complex chronic illnesses. Chief Medical Officer - The Non-Profit, The National Academy of Hypothyroidism and Integrative Sciences (NAHIS) NAHypothyroidism.org NAHIS is a non- profit, multidisciplinary medical society dedicated to the dissemination of new information on the diagnosis and treatment of hypothyroidism and complex conditions. NAHIS receives grants for clinical and laboratory research for novel methods in the diagnosis and treatment of hypothyroidism and chronic illnesses Chief Medical Officer - Integrative Peptides IntegrativePeptides.com Currently sells orally active and absorbable natural peptides in oral supplement form with unique delivery methods 2 Disclosure Statement Important Disclaimer In the interest of full disclosure, I am the All studies have limitations, and there is no perfect study. As with other studies, the studies Chief Medical Officer of Integrative Peptides. mentioned in this presentation and during the summit have limitations that should be considered when evaluating the efficacy of any treatment, including peptides, and determining the The opinions expressed are mine and do not appropriateness of peptide therapy for consumer use. A short summary, the abstract or reference to necessarily reflect those of Integrative a study may be discussed or provided. We are happy to provide you the entire study for your review of any study discussed, mentioned, presented or referenced. -

PEPTIDE INFORMATION Contact Kitt Burkley for Pricing 855-762-4878
PEPTIDE INFORMATION Contact Kitt Burkley for pricing 855-762-4878 AOD 9604 AOD 9604 works by mimicking the way natural HGH regulates fat metabolism but without the adverse effects on blood sugar or growth that is seen with unmodified HGH. Studies have suggested that AOD 9604 has an ability to stimulate lipolysis and inhibit lipogenesis. In addition AOD 9604 possesses other regenerative properties associated with growth hormone. Trials have been undertaken to determine the possible application of AOD 9604 in osteoarthritis and hypercholesterolemia, as well as for bone and cartilage repair. Results of oral glucose tolerance testing have demonstrated that AOD 9604 has no negative effect on carbohydrate metabolism, and it does not affect serum IGF-1 levels. AOD 9604 has an excellent safety profile and is generally well tolerated. It has attained Human GRAS status in the US. AOD 9604 600mcg/ml Cream – 30g AOD 9604* 0.5mg Troche BPC-157 (Body Protection Compound) BPC-157 promotes muscle and tendon healing, increases angiogenesis and decreases inflammatory response. Produces more type 1 collagen and has excellent potential for diabetic wound healing. BPC- 157 is highly stable, resistant to both hydrolysis and enzyme digestion. It can be given orally as well as administered subcutaneously or intramuscularly – no carrier molecule is required. This peptide has significant anti-inflammatory modulating effects. BPC-157 promotes tissue healing through signaling pathways – it provides specific response to particular injury thru cell signaling. BPC- 157 can be used continuously as body protective, or for specific period to treat body injury. BCP-157 is in clinical trials for IBD.