ANNUAL REPORT 2012 Contents OUR Mission
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
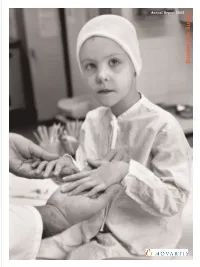
Caring and Curing
Caring and Curing Annual Report 2002 Novartis www.novartis.com Annual Report 2002 …molecules for medicines… Martin Klumpp, PhD, Lukas Leder, PhD, and Aline Tirat, Novartis Lead Discovery Center, Basel, Switzerland ...writing in sand... Sarvodaya Pre-School, Paluchera, Sri Lanka Contents Financial Highlights2 News in 20023 Letter from Daniel Vasella5 Amazing Patient Stories9 Division and Product Review Pharmaceuticals14 Joining Forces in the Quest for Cures22 Consumer Health29 Corporate Citizenship37 Animal Welfare46 Health, Safety and Environment49 Human Resources60 Corporate Governance Corporate Governance67 Board of Directors79 Executive Committee82 Business Unit Heads84 Financial Report Operating and Financial Review87 Equity Strategy and Share Information98 Group Consolidated Financial Statements and Notes104 Principal Companies138 Reconciliation to US GAAP140 Financial Statements of Novartis AG151 Due Dates for Reporting and Contacts157 Front cover: Svenja, a young eczema patient during an examination at the Center for Dermatology, University of Frankfurt am Main, Germany. Back cover: Svenja with her brother, Jan Patrick in the pediatric ward. Novartis Group 1 Financial Highlights Sales CHF millions Free cash flow2 CHF millions 2002 32 412 2002 4 463 2001 31 643 2001 4 073 20001 29 112 20001 3 254 Operating income CHF millions Research and development CHF millions 2002 7 887 2002 4 339 2001 7 277 2001 4 189 20001 6 727 20001 4 011 Net income CHF millions Employees at year end 2002 7 313 2002 72 877 2001 7 024 2001 71 116 20001 6 -

View the Gala Program
Global Citizen Award presented to Professor Klaus Schwab September 23, 2010 The Plaza • New York International Advisory Board Lt Gen Brent Scowcroft, USAF (Ret.), Chairman National Security Advisor to Presidents Gerald Ford and George H. W. Bush Senator Chuck Hagel Mr. Thomas H. Glocer The Rt. Hon. Lord Robertson of Chairman, Atlantic Council of the CEO, Thompson Reuters Port Ellen United States Mr. Francisco Gonzalez Former Secretary General of NATO Mr. Frederick Kempe Chairman & CEO, BBVA Ms. Güler Sabanci President & CEO, Atlantic Council Dr. James Goodnight Chairman & Managing Director, Members: Chief Executive Officer, SAS Haci Ömer Sabanci Holding A.S. Dr. Josef Ackermann Mr. Muhtar Kent Mr. Stephen A. Schwarzman Chairman of the Management Board Chairman & CEO, Chairman, CEO & Co-Founder, & the Group Executive Committee, The Coca-Cola Company The Blackstone Group Deutsche Bank AG President Aleksander Kwasniewski Sir Martin Sorrell H.E. Shaukat Aziz Former President of Poland Group Chief Executive, WPP Group PLC Former Prime Minister of Pakistan Mr. Jean Lemierre President Jose Maria Aznar Former President, The European Bank Mr. Robert J. Stevens Former Prime Minister of Spain for Reconstruction and Development Chairman, President & CEO, Lockheed Martin Dr. Zbigniew Brzezinski Mr. Alexey A. Mordashov National Security Advisor to President CEO, Severstal Mr. Sergey A. Taruta Jimmy Carter Chairman of the Board, Mr. Bob Moritz Industrial Union of Donbass Mr. Victor L. L. Chu U.S. Chairman & Senior Partner, Chairman, First Eastern Investment PricewaterhouseCoopers Dr. Gunter Thielen Group Chairman of the Supervisory Board, Mr. Rupert Murdoch Bertelsmann AG Mr. Richard Edelman Chairman & CEO, News Corporation President & CEO, Edelman Mr. -

ANNUAL REPORT 2012 Contents OUR Mission
ANNUAL REPORT 2012 cONTENTs OUR missiON GROUP REviEW Financial Highlights 2 We want to discover, develop and News in 2012 3 successfully market innovative Letter from Daniel vasella 5 products to prevent and cure interview with Joseph Jimenez 11 diseases, to ease suffering and HEALTHcAREPORTFOLiO contents 17 to enhance the quality of life. Pharmaceuticals 21 Novartis institutes for Biomedical Research 33 We also want to provide a Alcon 37 shareholder return that reflects sandoz 47 outstanding performance and vaccines and Diagnostics 53 to adequately reward those consumer Health 59 who invest ideas and work in cORPORATE REsPONsiBiLiTy contents 65 our company. Expanding Access to Healthcare 67 Doing Business Responsibly 77 independent Assurance Report 85 cORPORATE GOvERNANcE contents 87 Our Board of Directors 96 Our management 110 cOmPENsATiON REPORT contents 121 compensation Report 122 NOvARTis GROUP FiNANciAL REPORT contents 147 Financial Highlights 2012 148 Key Financial Developments 149 Operating and Financial Review 150 shareinformation 168 summary of Key Financial Data 188 Novartis Group consolidated Financial statements 190 Financial statements of Novartis AG 258 Annual Report Photography and Films 278 Key Dates 2013, contact information 280 and Forward-Looking statements 1 GROUP REviEW Novartis provides healthcare solutions that address the evolving needs of patients and societies worldwide. Our portfolio focuses on broad areas of healthcare: pharmaceuticals, eye care, generics, vaccines, consumer-based OTc and animal health. FiNaNcial -

Novartis GB06 Engl
119863_Nova_001_002_ug_e.qxp 24.1.2007 13:10 Uhr Seite 1 WWW.NOVARTIS.COM REPORT 2006 ANNUAL ANNUAL REPORT 2006 119863_Nova_000_000_web_e.qxp 24.1.2007 13:19 Uhr Seite 1 1 OUR MISSION CONTENTS We want to discover, develop and successfully market GROUP REVIEW innovative products to prevent and cure diseases, Overview 2 to ease suffering and to enhance the quality of life. Letter from Daniel Vasella 5 We also want to provide a shareholder return that reflects OPERATIONAL REVIEW outstanding performance and to adequately Pharmaceuticals 12 Vaccines and Diagnostics 36 reward those who invest ideas and work in our company. Sandoz 42 Consumer Health 50 CORPORATE CITIZENSHIP Introduction 56 Commitment to Patients 61 Novartis Foundation for 68 Sustainable Development Commitment to Our People 71 Commitment to Health, 77 Safety and Environment Commitment to Ethical 84 Business Conduct Independent Assurance Report 89 Risk Management 91 CORPORATE GOVERNANCE Commitment to Corporate Governance 93 Board of Directors 116 Executive Committee 122 Business Unit Heads 125 NOVARTIS GROUP FINANCIAL REPORT 2006 Contents 127 Operating & Financial Review 130 Equity Strategy 153 Novartis Group Consolidated 156 Financial Statements Novartis AG Financial Statements 224 Key Dates 2007 230 Contact Addresses 231 INSIDE FRONT COVER: KAMEDA MEDICAL CENTER; KAMOGAWA CITY, CHIBA, JAPAN FRONT COVER: GRUPO DE APOIO AO ADOLESCENTE E À CRIANÇA COM CÂNCER; SÃO PAULO, BRAZIL 119863_Nova_000_000_web_e.qxp 24.1.2007 13:19 Uhr Seite 2 2 GROUP Novartis is a world leader in providing medicines to protect health, prevent and treat disease, and to improve well-being. Novartis is the only company with leadership positions in patented and generic pharmaceuticals, vaccines and over-the-counter medicines. -

World Economic Forum Annual Meeting
World Economic Forum Annual Meeting List of Participants As of 30 April 2013 Davos-Klosters, Switzerland, 23-27 January 2013 Ivonne A-Baki Minister for the Yasuní-ITT Initiative of Ecuador Svein Aaser Chairman of the Board Telenor ASA Norway Florencio B. Abad Secretary of Budget and Management of the Philippines Mhammed Abbad Founder Al Jisr Morocco Andaloussi Faisal J. Abbas Editor-in-Chief Al Arabiya News Channel, United Arab Emirates English Service Ali Abbasov Minister of Communication and Information Technologies of Azerbaijan Mustafa Partner and Chairman of the Executive The Abraaj Group United Arab Emirates Abdel-Wadood Committee Mohd Razali Abdul Chairman Peremba Group of Companies Malaysia Rahman Khalid Honorary Chairman Vision 3 United Arab Emirates Abdulla-Janahi Abdullah II Ibn Al King of the Hashemite Kingdom of Hussein Jordan Rovnag Abdullayev President SOCAR (State Oil Company Azerbaijan of the Azerbaijan Republic) Shinzo Abe Prime Minister of Japan Derek Aberle Executive Vice-President, Qualcomm Qualcomm USA Incorporated and Group President Asanga Executive Director Lakshman Kadirgamar Sri Lanka Abeyagoonasekera Institute for International Relations and Strategic Studies Reuben Abraham Executive Director, Centre for Emerging Indian School of Business India Markets Solutions Magid Abraham Co-Founder, President and Chief comScore Inc. USA Executive Officer Issa Abdul Salam Chairman and Chief Executive Officer Salam International Qatar Abu Issa Investment Ltd Aclan Acar Chairman of the Board of Directors Dogus Otomotiv AS -

Notice of 2021 Annual Meeting of Shareholders and Proxy Statement Creating More Smiles with Every Sip and Every Bite℠ Dear Fellow Pepsico Shareholders
Faster, Stronger, Better. Notice of 2021 Annual Meeting of Shareholders and Proxy Statement Creating More Smiles with Every Sip and Every Bite℠ Dear Fellow PepsiCo Shareholders: • Continue to drive SodaStream — a rapidly growing and profitable business — as a sustainable and personalized beverage system; • Pivot our resources to provide first-class service levels for our customers; and • Increase our presence and availability across packages and channels where consumers migrated, such as multipacks and e-commerce. In addition, we returned a total of $7.5 billion in cash to shareholders through dividends and share repurchases and, earlier this year, announced a 5% increase in our Ramon L. Laguarta annualized dividend per share, effective with the expected Chairman of the Board of Directors and June 2021 dividend payment — our 49th consecutive Chief Executive Officer annual dividend increase. I am pleased to invite you to our 2021 Annual Meeting These outcomes underscore our commitment to creating of Shareholders on Wednesday, May 5, 2021 at 9:00 smiles for our shareholders, whilst confirming the strength a.m. Eastern Daylight Time. In light of ongoing concern and resilience of our business and affirming that our regarding COVID-19, we have planned for this year’s strategy is the right one in good times and in bad. event to be virtual-only. You can access the meeting at I am very proud of what we have been able to achieve, www.virtualshareholdermeeting.com/PEP2021. whilst staying focused on the issues that matter to our We believe our strategy and our people allowed us to society and planet. -

True North.Pdf
ffirs.qxp 1/17/07 8:25 PM Page v True North Discover Your Authentic Leadership Bill George With Peter Sims Foreword by David Gergen John Wiley & Sons, Inc. ffirs.qxp 1/17/07 8:25 PM Page ii ffirs.qxp 1/17/07 8:25 PM Page i True North ffirs.qxp 1/17/07 8:25 PM Page ii ffirs.qxp 1/17/07 8:25 PM Page iii A WARREN BENNIS BOOK This collection of books is devoted exclusively to new and exemplary contributions to management thought and practice. The books in this series are addressed to thoughtful leaders, executives, and managers of all organizations who are struggling with and committed to responsible change. My hope and goal is to spark new intellectual capital by sharing ideas positioned at an angle to conventional thought—in short, to publish books that disturb the present in the service of a better future. ffirs.qxp 1/17/07 8:25 PM Page iv BOOKS IN THE WARREN BENNIS SIGNATURE SERIES Branden Self-Esteem at Work Mitroff, Denton A Spiritual Audit of Corporate America Schein The Corporate Culture Survival Guide Sample The Contrarian’s Guide to Leadership Lawrence, Nohria Driven Cloke, Goldsmith The End of Management and the Rise of Organizational Democracy Glen Leading Geeks Cloke, Goldsmith The Art of Waking People Up George Authentic Leadership Kohlrieser Hostage at the Table Rhode Moral Leadership ffirs.qxp 1/17/07 8:25 PM Page v True North Discover Your Authentic Leadership Bill George With Peter Sims Foreword by David Gergen John Wiley & Sons, Inc. -

Annual Report 2011 Our Mission
ANNUAL REPORT 2011 OUR MISSION We want to discover, develop and successfully market innovative products to prevent and cure diseases, to ease suffering and to enhance the quality of life. We also want to provide a shareholder return that refl ects outstanding performance and to adequately reward those who invest ideas and work in our company. NOVA R TIS GRO UP A NNU AL REP ORT 2011 CONTENTS HEALTHCARE PERSPECTIVES by photojournalist Eugene Richards GROUP REVIEW Financial Highlights 2 News in 2011 3 Letter from Daniel Vasella 4 Interview with Joseph Jimenez 8 LIFE IN A CIRCLE 12 HEALTHCARE PORTFOLIO Contents 16 Pharmaceuticals 18 Novartis Institutes for BioMedical Research 29 NO GUARANTEES 32 Alcon 34 NEAR-PERFECT VISION 38 Sandoz 40 JUST THE BEGINNING 44 Vaccines and Diagnostics 46 THE TROUBLESHOOTER 50 Consumer Health 52 AN ONSET OF MALARIA 56 CORPORATE CITIZENSHIP Contents 60 Citizenship at Novartis 62 SOMETHING BEYOND THEMSELVES 68 Commitment to Patients 70 Commitment to People and Communities 72 THE VILLAGES MILES AWAY 74 Commitment to the Environment 76 Commitment to Ethical Business Conduct 78 Independent Assurance Report 81 THE LIVES OF OTHER PEOPLE 82 CORPORATE GOVERNANCE Contents 86 Our Board of Directors 92 Our Management 102 I AM WHO I AM 110 COMPENSATION REPORT Contents 114 Compensation Report 115 A GIFT FOR LIVING 134 NOVARTIS GROUP FINANCIAL REPORT Contents 138 Operating and Financial Review 141 Equity Strategy 187 Novartis Group Consolidated Financial Statements 190 Financial Statements of Novartis AG 262 Acknowledgements and 282 Annual Report Photography Key Dates 2012, Contact Information 284 and Forward-Looking Statements 2 | GROUP REVIEW 16 | HEALTHCARE PORTFOLIO 60 | CORPORATE CITIZENSHIP 86 | CORPORATE GOVERNANCE 114 | COMPENSATION REPORT 138 | FINANCIAL REPORT 1 GROUP REVIEW Novartis provides healthcare solutions that address the evolving needs of patients and societies worldwide. -

Caring and Curing Annual Report 2004
CARING AND CURING artis Nov 2004 AL REPORT ANNUAL REPORT 2004 www.novartis.com ANNU 10 000-liter extraction vessel, Novartis pharmaceutical production; Basel, Switzerland azil á, Br ortaleza, Cear en's Hospital Albert Sabin; F Back cover: Children's Hospital Albert Sabin; Fortaleza, Ceará, Brazil Childr OUR MISSION We want to discover, develop and successfully market innovative products to cure diseases, to ease suffering and to enhance the quality of life. We also want to provide a shareholder return that reflects outstanding performance and to adequately reward those who invest ideas and work in our company. GROUP OVERVIEW 2 Financial Highlights 3 News in 2004 5 Letter from Daniel Vasella 10 Divisions and Business Units Pharmaceuticals 40 Consumer Health 50 Corporate Citizenship 52 Commitment to Patients 66 Commitment to Our People 72 Commitment to Health, Safety and Environment 79 Commitment to Business Conduct 85 Corporate Governance Commitment to Corporate Governance 104 Board of Directors 110 Executive Committee 112 Business Unit Heads FINANCIAL OVERVIEW 115 Financial Report 194 Key Dates for 2005 195 Contact Addresses Front cover: Shanghai Children's Medical Center, Shanghai Second Medical University; Shanghai, China NOVARTIS GROUP 1 FINANCIAL HIGHLIGHTS Keyfigures Net sales, operating income and net income (in USD millions unless indicated otherwise) (in USD millions) 30 000 2004 2003 Net sales 28 247 24 864 25 000 Operating income 6539 5 889 Net income 5767 5 016 20 000 Return on sales (%) 23.1 23.7 15 000 Research and development -

PHARMA 40 2007 Bill and Melinda Gates, Co-Chairs, the Gates Foundation
PHARMA 40 2007 Bill and Melinda Gates, co-chairs, the Gates Foundation As co-chairs of the Bill & Melinda Gates Foundation, Bill Foundation’s global health programme, said: ‘The and Melinda Gates have control of a fund of $31.9 billion. pharmaceutical industry plays a huge role in improving One of the foundation’s core missions is to tackle deadly global health, and we are proud to support several diseases such as malaria and tuberculosis. With this kind public-private partnerships working with industry. The of spending power and their determination to drive up pipeline of new drugs and vaccines for the developing global healthcare provision, the Gates’ influence on the world is stronger than before.’ pharma industry is unquestionable. Bill began his philanthropic efforts in earnest in 1994, ‘The Gates have largely abandoned their commercial when he created the William H Gates Foundation, which ventures to run their foundation, which is dedicated to focused on global health. Three years later, he and Melinda research aimed at eliminating serious infectious diseases,’ created the Gates Library Foundation, which worked to commented one of our judges. ‘Their example has moved bring public access computers with internet connections others with substantial wealth to follow their example. to public libraries in the US. The two groups merged in 1hence they are a powerful force for good.’ 2000 to form the Bill & Melinda Gates Foundation. The Gates meet with local, national, and international Joining Microsoft Corporation in 1987, Melinda oversaw grantees and partners to further the foundation’s goal the development of many of Microsoft’s multimedia of improving equity around the world. -

Geschäftsbericht 2005 E
CARING AND CURING ANNUAL REPORT 2005 CENTRE HOSPITALIER UNIVERSITAIRE (CTU); TOULOUSE, FRANCE GROUP REVIEW 1 OUR MISSION CONTENTS We want to discover, develop and success- NOVARTIS GROUP BUSINESS REVIEW fully market innovative products to cure GROUP REVIEW 2 FINANCIAL HIGHLIGHTS diseases, to ease suffering and to enhance 3 NEWS the quality of life. 5 LETTER FROM DANIEL VASELLA OPERATIONAL REVIEW 12 BUSINESS OVERVIEW: We also want to provide a shareholder PHARMACEUTICALS SANDOZ return that reflects outstanding performance CONSUMER HEALTH and to adequately reward those who invest 22 BUSINESS STORIES ideas and work in our company. CORPORATE CITIZENSHIP 48 INTRODUCTION 57 COMMITMENT TO PATIENTS 62 COMMITMENT TO OUR PEOPLE 67 COMMITMENT TO HEALTH, SAFETY AND ENVIRONMENT 74 COMMITMENT TO ETHICAL BUSINESS CONDUCT CORPORATE GOVERNANCE 79 COMMITMENT TO CORPORATE GOVERNANCE 98 BOARD OF DIRECTORS 104 EXECUTIVE COMMITTEE 108 BUSINESS UNIT HEADS NOVARTIS GROUP FINANCIAL REPORT 111 CONTENTS 114 OPERATING & FINANCIAL REVIEW 134 EQUITY STRATEGY 136 NOVARTIS GROUP CONSOLIDATED FINANCIAL STATEMENTS 198 NOVARTIS AG FINANCIAL STATEMENTS 204 KEY DATES 2006 205 CONTACT ADDRESSES FRONT COVER: NURSE AND CHILD; JINKA HOSPITAL; JINKA, ETHIOPIA 2 FINANCIAL HIGHLIGHTS GROUP REVIEW FINANCIAL HIGHLIGHTS KEY FIGURES NET SALES, OPERATING INCOME AND NET INCOME (in USD millions unless indicated otherwise) (in USD millions) 2005 2004 1 Net sales 32 212 28 247 35000 6 905 Operating income 6 289 30000 Return on sales (%) 21.4 22.3 25000 Net income 6 141 5 601 Research and -

Programme for Printing
Global Agenda World Economic Forum Annual Meeting 2013 Programme Davos-Klosters, Switzerland 23-27 January Programme Pillars Programme Programme and Sub-Pillars Icons Co-Chairs Leading Through Adversity Building Resilient Institutions Arts and Culture Frederico Curado, President and Chief Executive Officer, EMBRAER, Brazil; Global Risks Improving Decision-Making Chair/Co-Chair of the Governors for Aviation and Strengthening Personal Resilience BetaZone Travel for 2013 Forum Debate Muhtar A. Kent, Chairman of the Board and Chief Executive Officer, The Coca-Cola IdeasLab Restoring Economic Company, USA Dynamism An Insight, An Idea Huguette Labelle, Chair, Achieving Inclusive Prosperity Transparency International, Televised session Germany; Global Agenda Rebuilding Economic Confidence Council on Responsible Mineral Lunch/Dinner session Resources Management Unleashing Entrepreneurial Innovation Andrew N. Liveris, Chairman WorkShop/WorkStudio session and Chief Executive Officer, The Dow Chemical Company, Interpretation USA Strengthening Societal Resilience On the record Atsutoshi Nishida, Chairman of the Board, Toshiba Reinforcing Critical Systems Corporation, Japan Sign-up required Sustaining Natural Resources Axel A. Weber, Chairman of the Board of Directors, UBS, Establishing Shared Norms Switzerland World Economic Forum Annual Meeting 2013 - Programme 2 Tue | Wed | Thu | Fri | Sat 09.00 - 21.00 16.00 - 16.45 18.00 - 18.10 Congress Centre - Sanada Congress Centre - Congress Hall 1 registration 2 forum vision 3 welcoming address Registration Opens World Economic Welcoming Address Please pick up your badge at registration Forum Vision and by the Executive on Kurgartenstrasse. However, access to the Congress Centre will only begin at Mission Chairman 14.00. Professor Klaus Schwab invites all newcomers and interested participants to Simultaneous interpretation in all a briefing on the institution's strategic languages vision and latest initiatives.