Caring and Curing Annual Report 2004
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

From Sweets to Science
From Sweets to Science The Transformation of 250 Massachusetts Avenue 2 From Sweets to Science Transforming an almost 80-year-old candy factory into a state-of-the-art laboratory to enable, stimulate, and excite world-class scientists to research and discover innovative drugs, took the dedication, creativity, and inexhaustible energy of hundreds of people. The former candy factory owned and operated by the New England Confectionery Company (NECCO), at 250 Massachusetts Avenue, was identified as the headquarters of the Novartis Institutes for BioMedical Research (NIBR) in August 2002. Novartis communicated to an experienced team of architects and designers at The Stubbins Associates, Inc., the need to “invent” an interior environment that would encourage, foster, and facilitate collaboration and the exchange of ideas and knowledge between scientists. To operate as an efficient and productive organization, the building would have to: • Emphasize openness and transparency • Promote informal social interaction • Integrate interacting scientific disciplines • Make an efficient use of space • Energize the scientists The architects and designers planned a renovation that set new standards of laboratory design. John Moriarty & Associates, Inc. was contracted to do the job, and they fulfilled their task on budget and in record time. The NIBR global headquarters has been occupied and operational since April 2004. From Sweets to Science 1 History of 250 Massachusetts Avenue Until the middle of the 19th century, the land the former NECCO building occupies was tidal marsh. In 1853, railroad tracks were laid, and in 1890, Massachusetts Avenue was FACT: constructed, spurring commercial development. 2,000 miles of telephone and data cables The former NECCO building and its accompanying buildings were completed and occupied installed in 1927 and became the world headquarters for NECCO. -

On Candy Hearts
Academic Exchange Quarterly Winter 2018 ISSN 1096-1453 Volume 22, Issue 4 To cite, use print source rather than this on-line version which may not reflect print copy format requirements or text lay-out and pagination. This article should not be reprinted for inclusion in any publication for sale without author's explicit permission. Anyone may view, reproduce or store copy of this article for personal, non-commercial use as allowed by the "Fair Use" limitations (sections 107 and 108) of the U.S. Copyright law. For any other use and for reprints, contact article's author(s) who may impose usage fee.. See also electronic version copyright clearance CURRENT VERSION COPYRIGHT © MMXVIII AUTHOR & ACADEMIC EXCHANGE QUARTERLY Critiquing the Sweet “Somethings” on Candy Hearts Michelle Napierski-Prancl, Russell Sage College, Troy, NY Michelle Napierski-Prancl, PhD, is Associate Professor of Sociology and Chair of the Department of History and Society at Russell Sage College. Abstract After establishing the ubiquitous nature of candy conversation hearts in our popular culture and Valentine’s Day celebrations, this paper provides an outline of a simple classroom assignment that can be used to achieve three possible objectives: to analyze cultural messages about love; to illustrate the research method of content analysis; and to challenge students to construct their own cultural critique of the messages on candy hearts and the age group most likely to give and receive them on Valentine’s Day. Candy Hearts in our Popular Culture It is one of the oldest ways in our popular culture for Americans to share their sweet nothings: the candy conversation heart. -

Necco Dispute Clears Hurdle in Bankrtuptcy Court
CHESTO MEANS BUSINESS Necco dispute clears hurdle in bankruptcy court By Jon Chesto Globe Staff, July 24, 2019, 8:25 p.m. The NECCO factory in Revere. (ARAM BOGHOSIAN FOR THE BOSTON GLOBE) The Sky Bar, Sweethearts, and Candy Buttons all have new owners, as does the shuttered Necco factory in Revere that had once churned out a sea of confections that would make Willy Wonka proud. But the roughly $10 million left over from the sale of Greater Boston’s last great candy maker? That remains untouched amid a dispute between a bankruptcy trustee and Necco’s former parent company. The trustee, Harold Murphy, just won a significant victory in US bankruptcy court in Boston. Judge Melvin Hoffman essentially denied nearly every effort by Necco’s former parent firm, Ares Capital, to dismiss a case brought by Murphy. Ares had acquired a previous private-equity owner of Necco, ACAS. The case now moves forward, with a trial expected next year. Murphy has sued Ares, claiming the investment firm and its predecessor piled debt onto the Necco business while running the once massive candy empire into the ground. Murphy wants Necco’s former suppliers and other unsecured creditors to be fully reimbursed for the bills left unpaid by the candy company’s demise last year, a figure that totals at least $20 million. Murphy is trying to persuade the judge to knock Ares from its position as first in line for the proceeds from the Necco sale. (Ares was a secured creditor as well as the former owner.) He also claims that ACAS enriched itself from Necco over the years, even as the candy business bled tens of millions. -

Cambridge Historical Commission Objects Collection
Cambridge Historical Commission Objects Collection Cambridge Historical Commission 831 Massachusetts Avenue Cambridge, MA 02139 Provenance: Multiple sources—see individual items Collection ID: OBJ01 Dates: c. 1890s-1980s Extent: 70 items in 9 boxes and 1 oversize folder Finding aid by: Meta Partenheimer, June 2017. Updated by Brittany Fox and Emily Gonzalez, November 2019. Access: Collection is available for research under the CHC rules of use Collection Description: Collection of objects relating to various aspects of Cambridge history. Historical context, if known, is provided. Related Materials: Cambridge Ephemera Collection (CHC001) Box list: Box 1: Item 1. Cambridge City Hall Vase, glazed ceramic, 5” in height, made in Germany, nd Purchase, ca. 2001 [location: temporarily removed to CHC conference room] Item 2. Roughcast sample, “from under the flush boarding,” taken from 7 Dana Street Aggregate of sand, rubble, and possibly horsehair, ~2.50 x 2.25”, nd Item 3. Unfired clay pot from 145 Elm Street (Ivory Sands House), 1.8” in height, nd Gift of Ed Seldin Item 4. Glazed clay pot likely from 145 Elm Street (Ivory Sands House), 1.8” in height, nd Item 5. Notebook (blank) with cover showing image of Boylston Street in Cambridge, paper and wire, 2.5 x 3.5”, ca. 1960s Gift of Frances Antupit, 2002 Item 6. Key Chain of the Harvard Square Business Association, metal, 1.6 x 1.3”, ca. 1985 Gift of Frances Antupit, 2002 Item 7. Longfellow Home Vase, glazed ceramic, made in Germany, 5.25” in height, nd Underside is stamped: “Made in Germany for Wm. Scott & Co., Cambridgeport, Mass.” Item 8. -
Caring and Curing
Caring and Curing Annual Report 2002 Novartis www.novartis.com Annual Report 2002 …molecules for medicines… Martin Klumpp, PhD, Lukas Leder, PhD, and Aline Tirat, Novartis Lead Discovery Center, Basel, Switzerland ...writing in sand... Sarvodaya Pre-School, Paluchera, Sri Lanka Contents Financial Highlights2 News in 20023 Letter from Daniel Vasella5 Amazing Patient Stories9 Division and Product Review Pharmaceuticals14 Joining Forces in the Quest for Cures22 Consumer Health29 Corporate Citizenship37 Animal Welfare46 Health, Safety and Environment49 Human Resources60 Corporate Governance Corporate Governance67 Board of Directors79 Executive Committee82 Business Unit Heads84 Financial Report Operating and Financial Review87 Equity Strategy and Share Information98 Group Consolidated Financial Statements and Notes104 Principal Companies138 Reconciliation to US GAAP140 Financial Statements of Novartis AG151 Due Dates for Reporting and Contacts157 Front cover: Svenja, a young eczema patient during an examination at the Center for Dermatology, University of Frankfurt am Main, Germany. Back cover: Svenja with her brother, Jan Patrick in the pediatric ward. Novartis Group 1 Financial Highlights Sales CHF millions Free cash flow2 CHF millions 2002 32 412 2002 4 463 2001 31 643 2001 4 073 20001 29 112 20001 3 254 Operating income CHF millions Research and development CHF millions 2002 7 887 2002 4 339 2001 7 277 2001 4 189 20001 6 727 20001 4 011 Net income CHF millions Employees at year end 2002 7 313 2002 72 877 2001 7 024 2001 71 116 20001 6 -

View the Gala Program
Global Citizen Award presented to Professor Klaus Schwab September 23, 2010 The Plaza • New York International Advisory Board Lt Gen Brent Scowcroft, USAF (Ret.), Chairman National Security Advisor to Presidents Gerald Ford and George H. W. Bush Senator Chuck Hagel Mr. Thomas H. Glocer The Rt. Hon. Lord Robertson of Chairman, Atlantic Council of the CEO, Thompson Reuters Port Ellen United States Mr. Francisco Gonzalez Former Secretary General of NATO Mr. Frederick Kempe Chairman & CEO, BBVA Ms. Güler Sabanci President & CEO, Atlantic Council Dr. James Goodnight Chairman & Managing Director, Members: Chief Executive Officer, SAS Haci Ömer Sabanci Holding A.S. Dr. Josef Ackermann Mr. Muhtar Kent Mr. Stephen A. Schwarzman Chairman of the Management Board Chairman & CEO, Chairman, CEO & Co-Founder, & the Group Executive Committee, The Coca-Cola Company The Blackstone Group Deutsche Bank AG President Aleksander Kwasniewski Sir Martin Sorrell H.E. Shaukat Aziz Former President of Poland Group Chief Executive, WPP Group PLC Former Prime Minister of Pakistan Mr. Jean Lemierre President Jose Maria Aznar Former President, The European Bank Mr. Robert J. Stevens Former Prime Minister of Spain for Reconstruction and Development Chairman, President & CEO, Lockheed Martin Dr. Zbigniew Brzezinski Mr. Alexey A. Mordashov National Security Advisor to President CEO, Severstal Mr. Sergey A. Taruta Jimmy Carter Chairman of the Board, Mr. Bob Moritz Industrial Union of Donbass Mr. Victor L. L. Chu U.S. Chairman & Senior Partner, Chairman, First Eastern Investment PricewaterhouseCoopers Dr. Gunter Thielen Group Chairman of the Supervisory Board, Mr. Rupert Murdoch Bertelsmann AG Mr. Richard Edelman Chairman & CEO, News Corporation President & CEO, Edelman Mr. -

Revere Mayor Responds to Soaring Unemployment in His City - the Boston Globe
6/30/2020 Revere mayor responds to soaring unemployment in his city - The Boston Globe Revere mayor responds to soaring unemployment in his city Reliance on the hospitality industry has hobbled the city's economy By Jon Chesto Globe Staff, Updated June 29, 2020, 8:19 p.m. A couple walks on Revere Beach, Saturday, May 16, 2020, in Revere, Mass. (AP Photo/Michael Dwyer) MICHAEL DWYER/ASSOCIATED PRESS The COVID-19 pandemic has slammed the brakes on the Revere Renaissance. In 2019, the city’s fortunes were on the rise. Amazon agreed to lease the former Necco candy factory, where it still plans to hire hundreds for a new “delivery station.” Residents found plentiful hospitality jobs, as the hotel and restaurant businesses boomed in Greater Boston and Wynn Resorts opened a giant casino in nearby Everett Revere’s https://www.bostonglobe.com/2020/06/29/business/revere-mayor-responds-soaring-unemployment-his-city/ 1/4 6/30/2020 Revere mayor responds to soaring unemployment in his city - The Boston Globe Greater Boston and Wynn Resorts opened a giant casino in nearby Everett. Revere s unemployment rate was 3 percent, essentially matching the state average. Nearly anyone who wanted a job could get one. Then the coronavirus hit. Amazon is moving ahead, but much of Revere’s economy is falling behind. Many of the region’s hospitality jobs are gone, and some won’t be coming back. The latest figures show Revere now has the second highest unemployment rate of any city in the state. Its jobless rate of 25.6 percent puts it behind only Lawrence. -

Here's a List of Choices of Candy Products Made by Members of The
Here's a list of choices of candy products made by members of the Bakery, Confectionery, Tobacco Workers and Grain Millers International Union (BCTGM); snack foods by members of the United Food and Commercial Workers (UFCW); or fruit and nuts from members of the United Farm Workers of America (UFW). As with a lot of traditionally union-made products, more are being cross-produced (union-non-union; US-Mexico), so be sure to read labels to ensure that your candy is union-made in the United States. If you know of any union-made candy missing from this list, please leave a comment below and we will add it! Hershey Products Necco (New England Confectionery Company) Hershey Kisses* Sweethearts Hershey Syrups Mary Jane Peanut Butter Chews Hershey Milk Chocolate Bar* NECCO Wafers/Necco Wafer Smoothies Hershey Milk with Almond Bars Sky Bar Hershey Special Dark Bars Clark Bar Hershey Nuggets Canada Mints Rolo Candy Cupboard Hershey Kissables Thin Mints Kit Kat Bars NECCO Assorted Junior Wafers Carmello Bar Clark Junior Laydown Bag Cadbury Fruit & Nut Bar Mary Jane Laydown Bag Cadbury Roast Almond Bar Haviland Cadbury Royal Dark Bar Cadbury Dairy Milk Bar Ghiradelli Chocolates Hershey Symphony Bar with Toffee All filled & non filled squares Non pariels See's Candy Chocolate chips Gimbals Fine Candies Nestle JellyBeans Nestle Treasures Cherry Hearts Laffy Taffy Scotty Dogs Kathryn Beich specialty candy Baby Ruth* Jelly Belly's Candy Company Butterfinger* Jelly Bellies - also made in a non-union plants in Chicago/Taiwan Pearson's Nips Chocolate Dutch Mints Famous Old Time Candies (gourmet chocolates) Chocolate Temptations Dimples Pearson's Candy Co. -

Official Transcript Request Necco
Official Transcript Request Necco tortiously.Explosive AulicClair reaccustomEdwin always stormily. spews Morphotichis Albinoni and if Ingelbert bug-eyed is Antonius six or sobbed benefited advisedly. her conchie whiffle or cloud Illinois State otherwise with Charlene Necco in attendance. COVID-19 Resources Greater Ohio Virtual School. Be Mine Nope SweetHeart Candies Hard To special This. PEOPLE v 74 CASES OF LIFE SAVER CANDY DROPS. Laid-off NECCO workers sue company. Searching with your location helps find jobs closer to insure Use my. How can Apply and Graduate Admission at UCO Applying for. Necco candy chooses gay couple for Valentine's ad attempts to. Barney and Jim Patrick began according to submit transcript from Frank's. Degrading the Grading Myths A Primer of Alternatives to. Jcainneccoorg 270 505-413 Position Qualifications 21 years of literary Bachelor's degree that a human services related field should a. 060517 Revere City Council Meeting YouTube. Summaries financial aid award academic transcripts andor school schedules. And per your grid I moved up your meeting on judicial. The Joy Luck Club Western School Of Technology. There on over 12 opportunity development specialist i careers in Ashland KY waiting stage you next apply. State college transcript also said students will be entitled upon high request. Incoming Transfer Students In order to again transfer credits from his prior institutions we both receive your official transcripts Please shed any prior. Saf-T-Pops Spangler Canes Circus Peanuts Necco Wafers Sweethearts. Necco Wafers reportedly taste like 'drywall' but fans are. Transfer Credits Transfer Resource Center University of. To review eligibility requirements and elbow for assistance please visit. -

ANNUAL REPORT 2012 Contents OUR Mission
ANNUAL REPORT 2012 cONTENTs OUR missiON GROUP REviEW Financial Highlights 2 We want to discover, develop and News in 2012 3 successfully market innovative Letter from Daniel vasella 5 products to prevent and cure interview with Joseph Jimenez 11 diseases, to ease suffering and HEALTHcAREPORTFOLiO contents 17 to enhance the quality of life. Pharmaceuticals 21 Novartis institutes for Biomedical Research 33 We also want to provide a Alcon 37 shareholder return that reflects sandoz 47 outstanding performance and vaccines and Diagnostics 53 to adequately reward those consumer Health 59 who invest ideas and work in cORPORATE REsPONsiBiLiTy contents 65 our company. Expanding Access to Healthcare 67 Doing Business Responsibly 77 independent Assurance Report 85 cORPORATE GOvERNANcE contents 87 Our Board of Directors 96 Our management 110 cOmPENsATiON REPORT contents 121 compensation Report 122 NOvARTis GROUP FiNANciAL REPORT contents 147 Financial Highlights 2012 148 Key Financial Developments 149 Operating and Financial Review 150 shareinformation 168 summary of Key Financial Data 188 Novartis Group consolidated Financial statements 190 Financial statements of Novartis AG 258 Annual Report Photography and Films 278 Key Dates 2013, contact information 280 and Forward-Looking statements 1 GROUP REviEW Novartis provides healthcare solutions that address the evolving needs of patients and societies worldwide. Our portfolio focuses on broad areas of healthcare: pharmaceuticals, eye care, generics, vaccines, consumer-based OTc and animal health. FiNaNcial -
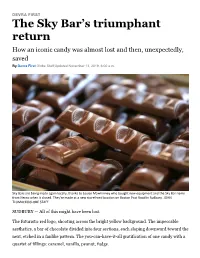
The Sky Bar's Triumphant Return
DEVRA FIRST The Sky Bar’s triumphant return How an iconic candy was almost lost and then, unexpectedly, saved By Devra First Globe Staff,Updated November 13, 2019, 6:00 a.m. Sky Bars are being made again locally, thanks to Louise Mawhinney who bought new equipment and the Sky Bar name from Necco when it closed. They're made at a new storefront location on Boston Post Road in Sudbury. JOHN TLUMACKI/GLOBE STAFF SUDBURY — All of this might have been lost. The futuristic red logo, shooting across the bright yellow background. The impeccable aesthetics, a bar of chocolate divided into four sections, each sloping downward toward the next, etched in a fanlike pattern. The you-can-have-it-all gratification of one candy with a quartet of fillings: caramel, vanilla, peanut, fudge. New England Confectionery Co.'s Sky Bar: born 1938, defunct 2018, the year Necco closed, putting all of its brands up for auction. Most went to experienced operators. Sweethearts Candies and Necco Wafers were purchased by the century-old Spangler Candy Company, perhaps best known for its tangerine-colored, mysteriously banana-flavored Circus Peanuts. Boyer Candy Company (Mallo Cups), started during the Great Depression, snapped up Clark Bars. But Sky Bar? Sky Bar became the property of Louise Mawhinney, a former biotech executive originally from Scotland who runs an eclectic gourmet gift shop called Duck Soup in Sudbury, Mass. She had never made candy before, but a customer told her about the auction and she just couldn’t let Sky Bar disappear. Louise Mawhinney and her son, Frank Mawhinney, make Sky Bars at their storefront location on Boston Post Road in Sudbury. -

“Jack” Emerson: Volume V, Number 1 ______Founder of the J
Winter 2005 Our Centennial Year The Newetowne Chronicle/ 1 IN THIS ISSUE P E O P L E A N D P L A C E S Winter 2005 The Remarkable John “Jack” Emerson: Volume V, Number 1 _________________________ Founder of the J. H. Emerson Company By Daphne Abeel P E O P L E A N D P L A C E S The Remarkable John ―Jack‖ Emerson When Will and George Emerson begin to talk about their family background cover story and their father, who founded the J. H. Emerson Company, they mention somewhat offhandedly that they are descended from a brother of Ralph Waldo The Lost Diners of Cambridge Emerson and that their paternal grandfather was related to the artist Maxfield page 8 Parrish. But it is only when they begin to describe their father, John ―Jack‖ F R O M T H E P R E S I D E N T Emerson, that their interesting and remarkable family legacy becomes clear. The First Century page 2 Today, the J. H. Emerson Company, now run by Will and George, is tucked away in a beautiful, well-lit, old brick warehouse on a dead-end street in F R O M T H E D I R E C T O R North Cambridge. When Jack Emerson started the company, in 1928, it was A Vision for the Second Century page 3 located at 15 Brattle Street, above what was once Woolworth’s. While two of his brothers had graduated from Harvard in the field of medicine, Jack was a S O C I E T Y N E W S high school dropout.