Bruktawit Desta Liben
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
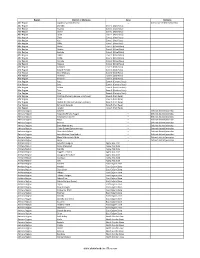
Districts of Ethiopia
Region District or Woredas Zone Remarks Afar Region Argobba Special Woreda -- Independent district/woredas Afar Region Afambo Zone 1 (Awsi Rasu) Afar Region Asayita Zone 1 (Awsi Rasu) Afar Region Chifra Zone 1 (Awsi Rasu) Afar Region Dubti Zone 1 (Awsi Rasu) Afar Region Elidar Zone 1 (Awsi Rasu) Afar Region Kori Zone 1 (Awsi Rasu) Afar Region Mille Zone 1 (Awsi Rasu) Afar Region Abala Zone 2 (Kilbet Rasu) Afar Region Afdera Zone 2 (Kilbet Rasu) Afar Region Berhale Zone 2 (Kilbet Rasu) Afar Region Dallol Zone 2 (Kilbet Rasu) Afar Region Erebti Zone 2 (Kilbet Rasu) Afar Region Koneba Zone 2 (Kilbet Rasu) Afar Region Megale Zone 2 (Kilbet Rasu) Afar Region Amibara Zone 3 (Gabi Rasu) Afar Region Awash Fentale Zone 3 (Gabi Rasu) Afar Region Bure Mudaytu Zone 3 (Gabi Rasu) Afar Region Dulecha Zone 3 (Gabi Rasu) Afar Region Gewane Zone 3 (Gabi Rasu) Afar Region Aura Zone 4 (Fantena Rasu) Afar Region Ewa Zone 4 (Fantena Rasu) Afar Region Gulina Zone 4 (Fantena Rasu) Afar Region Teru Zone 4 (Fantena Rasu) Afar Region Yalo Zone 4 (Fantena Rasu) Afar Region Dalifage (formerly known as Artuma) Zone 5 (Hari Rasu) Afar Region Dewe Zone 5 (Hari Rasu) Afar Region Hadele Ele (formerly known as Fursi) Zone 5 (Hari Rasu) Afar Region Simurobi Gele'alo Zone 5 (Hari Rasu) Afar Region Telalak Zone 5 (Hari Rasu) Amhara Region Achefer -- Defunct district/woredas Amhara Region Angolalla Terana Asagirt -- Defunct district/woredas Amhara Region Artuma Fursina Jile -- Defunct district/woredas Amhara Region Banja -- Defunct district/woredas Amhara Region Belessa -- -

Baliq: Indigenous Conflict Resolution Mechanism
NOVATEUR PUBLICATIONS INTERNATIONAL JOURNAL OF INNOVATIONS IN ENGINEERING RESEARCH AND TECHNOLOGY [IJIERT] ISSN: 2394-3696 Website: ijiert.org VOLUME 8, ISSUE 1, Jan.-2021 BALIQ: INDIGENOUS CONFLICT RESOLUTION MECHANISM AMONG THE SILTE PEOPLE: THE CASE OF SILTI WOREDA, KIBET TOWN KEBEDE LEMU BEKELCHA Lecturer of Social Anthropology, Bule Hora University, Oromia, Ethiopia, Email: [email protected] AREGASH ETICHA SEFERA Lecturer of Social Anthropology, Bule Hora University Oromia, Ethiopia, Email: [email protected] LENSA TUFA FOGI Lecturer of Social Anthropology, Bule Hora University, Oromia, Ethiopia, and Email: [email protected] ABSTRACT The different ethnic groups in Ethiopia have developed their own indigenous mechanism to deal with conflicts. The Silte of southern Ethiopia is among those ethnic groups with their own conflict resolution mechanism. The main purpose of this study is to explore the indigenous institutions of conflict resolution among the Silte people of southern Ethiopia. This study has employed a qualitative research to meet the stated objectives of the study. To achieve the above objectives, the study collected primary data from different informants in Silte Woreda by employing such qualitative data collection techniques as an interview, focus group discussions and observation. The secondary data sources were obtained from published and unpublished government documents; such as books and magazine and. The finding of this research reveals that the indigenous conflict resolution institution is one of the ways of resolving conflict in the study area. The study also came up with the major causes of conflict in the study area and the indigenous means to resolve them through the Baliq conflict resolution institution. -

The Invest Habitat Quality Model Associated with Land Use/Cover Changes: a Qualitative Case Study of the Winike Watershed in the Omo-Gibe Basin, Southwest Ethiopia
remote sensing Article The InVEST Habitat Quality Model Associated with Land Use/Cover Changes: A Qualitative Case Study of the Winike Watershed in the Omo-Gibe Basin, Southwest Ethiopia Abreham Berta Aneseyee 1,2 , Tomasz Noszczyk 3,* , Teshome Soromessa 1 and Eyasu Elias 1 1 Center of Environmental Science, College of Natural and Computational Sciences, Addis Ababa University, Addis Ababa, P.O. Box 1176, Ethiopia; [email protected] (A.B.A.); [email protected] (T.S.); [email protected] (E.E.) 2 Department of Natural Resource Management, Wolkite University, Ethiopia, Wolkite, P.O. Box 07, Ethiopia 3 Department of Land Management and Landscape Architecture, Faculty of Environmental Engineering and Land Surveying, University of Agriculture in Krakow, Krakow, Poland, 253c Balicka Street, 30-149 Krakow, Poland * Correspondence: [email protected] Received: 17 February 2020; Accepted: 27 March 2020; Published: 30 March 2020 Abstract: The contribution of biodiversity to the global economy, human survival, and welfare has been increasing significantly, but the anthropogenic pressure as a threat to the pristine habitat has followed. This study aims to identify habitat suitability, analyze the change in habitat quality from 1988 to 2018, and to investigate the correlation between impact factors and habitat quality. The InVEST habitat quality model was used to analyze the spatiotemporal change in habitat quality in individual land-use types in the Winike watershed. Remote sensing data were used to analyze the land use/land cover changes. Nine threat sources, their maximum distance of impact, mode of decay, and sensitivity to threats were also estimated for each land-use cover type. -

The Case of Sasakawa Global 2000 Ethiopia in Gumer Woreda By
Addis Ababa University School of Graduate Studies College of Business and Economics Department of Public Administration and Development Management Assessing the role of Development partners on agricultural extension delivery: The case of Sasakawa Global 2000 Ethiopia in Gumer woreda By: Temesgen Tamrat Advisor: Mulugeta Abebe (PH.D) A thesis submitted to the School of Graduate Studies of Addis Ababa University in partial fulfillment of the requirements for the Degree of Masters in Public Management and Policy (MPMP) Specialized In Development Management June, 2017 Addis Ababa, Ethiopia Addis Ababa University School of Graduate Studies College of Business and Economics Department of Public Administration and Development Management This is to certify that the thesis prepared by Temesgen Tamrat Yohans entitled “Assessing the role of Development partners on agricultural extension delivery: The case of Sasakawa Global 2000 Ethiopia in Gumer woreda”, which is submitted in partial fulfillment of the requirements for the Degree of Public Management and Policy (MPMP), complies with the regulations of the University and meets the accepted standards with respect to standards to originality and quality. Approved by Board of Examiners: Mulugeta Abebe (PhD) ___________________ _______________ Advisor Signature Date Elias Berhanu (PhD) ___________________ _________________ Internal Examiner Signature Date Flimon Handaro (PhD) ___________________ _______________ External Examiner Signature Date Declaration Student ID: GSE/0632/06 I declare that this research report on ‘Assessing the role of Development partners on agricultural extension delivery:The case of Sasakawa Global 2000 Ethiopia in Gumer woreda’ is my own original work with assistances and guidance from my advisor and not submitted before for any institution and any purpose. -

A History of a Reaction of the Mareko People Against the Italian Invasion and the Five Year Italian Rule-In South Central Ethiopia
Historical Research Letter www.iiste.org ISSN 2224-3178 (Paper) ISSN 2225-0964 (Online) Vol.53, 2021 A History of a Reaction of the Mareko People Against the Italian Invasion and the Five Year Italian Rule-in South Central Ethiopia Yohannes Tesfsye Getachew * Buruk Woldemichael Jima Department of Histroy and Heriatge Management, Jimma University, Jimma, PO box 378, Ethiopia Abstract The prime objective of this paper is to explore a history of reaction made by Mareko people against the Italian invasion and the five year occupation. The paper also uncovers the role played by Mareko and other integrated ethno-linguistic individual patriots who fight against Italian occupation and rule at the then Mareko woreda (district). At the eve of the Fascist Italian aggression Mareko people were lived under Mareko woreda (district) which was under the administrative division of Shawa tekely gezat (division of country). Butajira town was the administrative site of the then Mareko woreda. Like other nation, nationalities, and peoples of Ethiopia, fighting class from Mareko people marched in Maychew and other battles to defend their county from Italian aggression. Even though the final battle at Maychew was unsuccessful, they actively resist Italian rule. To speak frankly the then governor of Dobena Sub- district and his officers peacefully submitted and became a leading collaborator, but the majority of Mareko people resisted for the Italian rule. The resistance was mainly led by Wärѐqѐ Märeyamѐ, spiritual leader of Mareko people in Mareko land, and qegnazmach Tuji Anjilo outside Mareko land. Subsequently the Italian Fascist officials established military camp at Koshe kebele the center of Dobena sub- district. -

Modeling Malaria Cases Associated with Environmental Risk Factors in Ethiopia Using Geographically Weighted Regression
MODELING MALARIA CASES ASSOCIATED WITH ENVIRONMENTAL RISK FACTORS IN ETHIOPIA USING GEOGRAPHICALLY WEIGHTED REGRESSION Berhanu Berga Dadi i MODELING MALARIA CASES ASSOCIATED WITH ENVIRONMENTAL RISK FACTORS IN ETHIOPIA USING THE GEOGRAPHICALLY WEIGHTED REGRESSION MODEL, 2015-2016 Dissertation supervised by Dr.Jorge Mateu Mahiques,PhD Professor, Department of Mathematics University of Jaume I Castellon, Spain Ana Cristina Costa, PhD Professor, Nova Information Management School University of Nova Lisbon, Portugal Pablo Juan Verdoy, PhD Professor, Department of Mathematics University of Jaume I Castellon, Spain March 2020 ii DECLARATION OF ORIGINALITY I declare that the work described in this document is my own and not from someone else. All the assistance I have received from other people is duly acknowledged, and all the sources (published or not published) referenced. This work has not been previously evaluated or submitted to the University of Jaume I Castellon, Spain, or elsewhere. Castellon, 30th Feburaury 2020 Berhanu Berga Dadi iii Acknowledgments Before and above anything, I want to thank our Lord Jesus Christ, Son of GOD, for his blessing and protection to all of us to live. I want to thank also all consortium of Erasmus Mundus Master's program in Geospatial Technologies for their financial and material support during all period of my study. Grateful acknowledgment expressed to Supervisors: Prof.Dr.Jorge Mateu Mahiques, Universitat Jaume I(UJI), Prof.Dr.Ana Cristina Costa, Universidade NOVA de Lisboa, and Prof.Dr.Pablo Juan Verdoy, Universitat Jaume I(UJI) for their immense support, outstanding guidance, encouragement and helpful comments throughout my thesis work. Finally, but not least, I would like to thank my lovely wife, Workababa Bekele, and beloved daughter Loise Berhanu and son Nethan Berhanu for their patience, inspiration, and understanding during the entire period of my study. -

Limnologica 65 (2017) 61–75
Limnologica 65 (2017) 61–75 Contents lists available at ScienceDirect Limnologica journal homepage: www.elsevier.com/locate/limno Farmers’ awareness and perception of Lake Ziway (Ethiopia) and its MARK watershed management ⁎ Hayal Destaa,b, , Brook Lemmab,c, Till Stellmacherd a Rachel Carson Center for Environment and Society, Ludwig-Maximilians-University (LMU), Leopoldstr. 11a, D-80802, Munich, Germany b Chair of Ecosystem Planning and Management, EiABC, Addis Ababa University, P. O. Box 518, Addis Ababa, Ethiopia c Department of Zoological Sciences, College of Natural Science, Addis Ababa University, P. O. Box 1176, Addis Ababa, Ethiopia d Center for Development Research (ZEF), University of Bonn, Walter-Flex-Str. 3, D-53113, Bonn, Germany ARTICLE INFO ABSTRACT Keywords: The article examines how heads of farmers’ households perceive the socioeconomic benefits of Lake Ziway Watershed management (Ethiopia), the causes of its current degradation, and the state of land and water use management in its wa- Local perception tershed. The investigation was based on in-depth empirical field work including a survey with 635 heads of Awareness smallholder farmers’ households via interview using semi-structured questionnaires. Further, water abstraction Lake was estimated from three districts that border with the lake. Respondents believe that Lake Ziway provides a Water abstraction number of individual and collective benefits for local communities, private companies and public institution. They stated, however, that the lake is under pressure from the floriculture industry and other investment pro- jects, high population growth and subsequent expansion of settlements and irrigation farms, high applications of agrochemicals, soil erosion, uncontrolled water abstraction, and deforestation in the watershed. -

Awareness of Community on Fishery and Aquaculture Production in Central Ethiopia
Alemu A. J Aquac Fisheries 2021, 5: 039 DOI: 10.24966/AAF-5523/100039 HSOA Journal of Aquaculture & Fisheries Research Article The domestic fishery of Africa involvement is projected to be Awareness of Community about 2.1 million tons of fish per year; it epitomizes 24% of the total world fish production from inland water bodies. The inland water on Fishery and Aquaculture body of Ethiopia is enclosed about 7,400 km2 of the lakes and about 7,000 km a total length of the rivers [2]. Further, 180 fish species were Production in Central Ethiopia harbored in these water bodies [3]. In Ethiopia, fish comes exclusively from inland water bodies with lakes, rivers, streams, reservoirs and substantial wetlands that are of great socio-economic, ecological and Tena Alemu * scientific importance [4,5]. Department of Animal Production and Technology, Wolkite University, Wol- kite, Ethiopia Ethiopia being a land locked country its fisheries is entirely based on inland water bodies, lakes, reservoirs and rivers. Fish production potential of the country is estimated to be 51,400 tonnes per annum [6]. Fishing has been the main source of protein supply for many Abstract people particularly for those who are residing in the locality of major water bodies like Lake Tana, Ziway, Awassa, Chamo, Baro River, etc The study was conducted in three different districts Gumer, [5]. Ethiopia is capable with numerous water bodies that cover a high Enemornaener and Cheha Woreda on awareness and perception of community on fishery and aquaculture production. In those diversity of aquatic wildlife. Reservoir fishery plays an important study areas majority of the people had the limitation of knowledge role in the economy of the country and the livelihoods of the people on production, consumption, and use of fish and aquaculture living adjacent to those reservoirs. -

(Gurage, Mareqo, Qebena, and Silti), South Central Ethiopia Alemtshay Teka1*, Zemede Asfaw2, Sebsebe Demissew2 and Patrick Van Damme3,4
Teka et al. Journal of Ethnobiology and Ethnomedicine (2020) 16:27 https://doi.org/10.1186/s13002-020-00377-1 RESEARCH Open Access Medicinal plant use practice in four ethnic communities (Gurage, Mareqo, Qebena, and Silti), south central Ethiopia Alemtshay Teka1*, Zemede Asfaw2, Sebsebe Demissew2 and Patrick Van Damme3,4 Abstract Background: Ethnic groups throughout the world have developed their own cultures expressed in the form of customs, taboos, and traditional healthcare systems. Traditional medicine system is one of the widespread cultures known throughout the world which is very much tied to cultural practices of the community or ethnic group. Medicinal plant treasure found in Gurage and Silti zones remained poorly characterized and understood. Therefore, this study was conducted in four ethnic groups: three from Gurage zone (Gurage, Qebena, and Mareqo) and one from Silti zone (Silti) which have lived in close proximity and contact for many centuries in the respective zones. In the present study, unique and shared cultural elements in connection to traditional herbal medicine were examined through investigation of the diversity of medicinal plants. Moreover, attempts have been made to determine similarities among the society in the medicinal plants they have used in general and in medicinal plant species considered culturally most important. Methods: In a study that involved 320 randomly sampled informants, semi-structured interviews, focus group discussions, and participant observation were used and qualitative and quantitative data were collected. Descriptive statistics, rank order priority (ROP), informant consensus factor, Jaccard similarity coefficient, and clustering were used for data analysis. Results: A total of 244 medicinal plant species and a fungal species used to treat human and/or livestock ailments were documented. -

World Bank Document
Sample Procurement Plan (Text in italic font is meant for instruction to staff and should be deleted in the final version of the PP) Public Disclosure Authorized (This is only a sample with the minimum content that is required to be included in the PAD. The detailed procurement plan is still mandatory for disclosure on the Bank’s website in accordance with the guidelines. The initial procurement plan will cover the first 18 months of the project and then updated annually or earlier as necessary). I. General 1. Bank’s approval Date of the procurement Plan: Updated Procurement Plan, M 2. Date of General Procurement Notice: Dec 24, 2006 Public Disclosure Authorized 3. Period covered by this procurement plan: The procurement period of project covered from year June 2010 to December 2012 II. Goods and Works and non-consulting services. 1. Prior Review Threshold: Procurement Decisions subject to Prior Review by the Bank as stated in Appendix 1 to the Guidelines for Procurement: [Thresholds for applicable procurement methods (not limited to the list below) will be determined by the Procurement Specialist /Procurement Accredited Staff based on the assessment of the implementing agency’s capacity.] Public Disclosure Authorized Procurement Method Prior Review Comments Threshold US$ 1. ICB and LIB (Goods) Above US$ 500,000 All 2. NCB (Goods) Above US$ 100,000 First contract 3. ICB (Works) Above US$ 15 million All 4. NCB (Works) Above US$ 5 million All 5. (Non-Consultant Services) Below US$ 100,000 First contract [Add other methods if necessary] 2. Prequalification. Bidders for _Not applicable_ shall be prequalified in accordance with the provisions of paragraphs 2.9 and 2.10 of the Public Disclosure Authorized Guidelines. -

Knowledge of Health Professionals on Cold Chain Management and Associated Factors in Ezha District, Gurage Zone, Ethiopia
Hindawi Scientifica Volume 2019, Article ID 6937291, 7 pages https://doi.org/10.1155/2019/6937291 Research Article Knowledge of Health Professionals on Cold Chain Management and Associated Factors in Ezha District, Gurage Zone, Ethiopia Zeyneba Jemal Yassin,1 Habtamu Yimer Nega,2 Behailu Tariku Derseh ,3 Yetnayet Sisay Yehuala,4 and Abel Fekadu Dad 5,6 1Ethiopian Field Epidemiology and Laboratory Training Program Resident, University of Gondar, Gondar, Ethiopia 2South Wollo Zone, Institute of Public Health Emergency Management Department, Dessie, Ethiopia 3Debre Berhan University, College of Health Sciences, Department of Public Health, Debre Berhan, Ethiopia 4University of Gondar, Institute of Public Health, Department of Health Promotion and Behavioral Sciences, Gondar, Ethiopia 5College of Medicine and Public Health, School of Public Health, Flinders University, Adelaide, Australia 6University of Gondar, Institute of Public Health, Department of Epidemiology Biostatistics, Gondar, Ethiopia Correspondence should be addressed to Abel Fekadu Dad; [email protected] Received 26 December 2018; Revised 3 April 2019; Accepted 22 April 2019; Published 9 June 2019 Academic Editor: Haiqi He Copyright © 2019 Zeyneba Jemal Yassin et al. -is is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. Background. Maintaining quality of vaccines has been one of the main challenges of immunization programs in Africa including Ethiopia, and this could mainly be explained by health professional’s knowledge about cold chain management. -ere are limited studies done in Ethiopia linking the knowledge of health professionals on cold chain management, and that is why we needed to conduct this study. -

WCBS III Supply Side Report 1
Federal Democratic Republic of Ethiopia Ministry of Capacity Building in Collaboration with PSCAP Donors "Woreda and City Administrations Benchmarking Survey III” Supply Side Report Survey of Service Delivery Satisfaction Status Final Addis Ababa July, 2010 ACKNOWLEDGEMENT The survey work was lead and coordinated by Berhanu Legesse (AFTPR, World Bank) and Ato Tesfaye Atire from Ministry of Capacity Building. The Supply side has been designed and analysis was produced by Dr. Alexander Wagner while the data was collected by Selam Development Consultants firm with quality control from Mr. Sebastian Jilke. The survey was sponsored through PSCAP’s multi‐donor trust fund facility financed by DFID and CIDA and managed by the World Bank. All stages of the survey work was evaluated and guided by a steering committee comprises of representatives from Ministry of Capacity Building, Central Statistical Agency, the World Bank, DFID, and CIDA. Large thanks are due to the Regional Bureaus of Capacity Building and all PSCAP executing agencies as well as PSCAP Support Project team in the World Bank and in the participating donors for their inputs in the Production of this analysis. Without them, it would have been impossible to produce. Table of Content 1 Executive Summary ...................................................................................................... 1 1.1 Key results by thematic areas............................................................................................................ 1 1.1.1 Local government finance ...................................................................................................