Prothrombin Time Testing Introduction the Prothrombin Time (PT) Was First Described by Quick Et Al
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
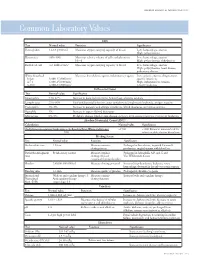
Common Laboratory Values
AmericAn AcAdemy of PediAtric dentistry Reference Manual 2006-2007 Resource Section 251 Common LaboratoryCommon Values Laboratory Values CBC Test Normal value Function Significance Hemoglobin 12-18 g/100 mL Measures oxygen carrying capacity of blood Low: hemorrhage, anemia High: polycythemia Hematocrit 35%-50% Measures relative volume of cells and plasma in Low: hemorrhage, anemia blood High: polycythemia, dehydration Red blood cell 4-6 million/mm3 Measures oxygen-carrying capacity of blood Low: hemorrhage, anemia High: polycythemia, heart disease, pulmonary disease White blood cell Measures host defense against inflammatory agents Low: aplastic anemia, drug toxicity, Infant 8,000-15,000/mm3 specific infections 4-7 y 6,000-15,000/mm3 High: inflammation, trauma, 8-18 y 4,500-13,500/mm3 toxicity, leukemia Differential Count Test Normal value Significance Neutrophils 54%-62% Increase in bacterial infections, hemorrhage, diabetic acidosis Lymphocytes 25%-30% Viral and bacterial infection, acute and chronic lymphocytic leukemia, antigen reaction Eosinophils 1%-3% Increase in parasitic and allergic conditions, blood dyscrasias, pernicious anemia Basophils 1% Increase in types of blood dyscrasias Monocytes 0%-9% Hodgkin’s disease, lipid storage disease, recovery from severe infections, monocytic leukemia Absolute Neutrophil Count (ANC) Calculation Normal value Significance (% Polymorphonuclear Leukocytes + % Bands)×Total White Cell Count >1500 <1000 Patient at increased risk for 100 infection; defer elective dental care Bleeding Screen Test -

Coagulation Testing: High Hematocrit-Anticoagulant Adjustment
PREANALYTIC PULSE Coagulation Testing: High Hematocrit-Anticoagulant Adjustment In 1980, CLSI released H21-A4 guidelines for coagulation testing, which included the recommendation to adjust/correct the amount of citrate in blue-top evacuated blood collection tubes for patients presenting hematocrits greater than 55%. In addition to the CLSI guidelines, the CAP hematology checklist HEM.22830 includes the question, “Are there documented guidelines for the detection and special handling of specimens with elevated hematocrits?” Principle The adjustment is to assure the plasma:anticoagulant ratio (not the blood:anticoagulant ratio) stays consistent. A patient with high hematocrit, greater than 55%, will result in less plasma after centrifugation. The plasma fraction will contain an increased concentration of sodium citrate anticoagulant. The increased concentration may result in falsely prolonged test results for Prothrombin Time (PT) and Activated Partial Thromboplastin Time (aPTT). Formula 1. To calculate the corrected 3.2% sodium citrated whole blood for hematocrit > 55%, adjust the citrate to the proper volume with the following formula: -3 C = (1.85 x 10 )(100-HCT)(Vblood) Where: C = volume of sodium citrate required for that volume of blood HCT = patient’s hematocrit V = volume of blood required in the blood collection tube (example if a 3mL tube is used the blood draw volume is 2.7mL) 1.85 x 10-3 is a constant (considering the citrate volume, blood volume, and citrate concentration). 2. Example: Patient has a Hct of 60% and the patient’s blood will be drawn into a 3mL VACUETTE® sodium citrate (blue-top) blood collection tube. Adjustment of the sodium citrate volume is calculated as: C = (1.85 x 10-3)(100-60)(2.7mL) C = 0.20mL (rounded up from 01.998mL) Remove: 0.30 - 0.20 = 0.10mL of sodium citrate to be removed (0.30 is the difference between the total tube volume of 3.0mL and the blood drawn into the tube of 2.7mL) Method The instructions to prepare an adjusted sodium citrate tube are to be documented in the Laboratory Standard Operating Procedures. -

Role of Real-Time / Accurate Diagnostic Testing in Patient Blood Management Programs
ROLE OF REAL-TIME / ACCURATE DIAGNOSTIC TESTING IN PATIENT BLOOD MANAGEMENT PROGRAMS POINT-OF-CARE TESTING “Diagnostic testing is an essential component of Patient Blood Management. The accurate assessment of the true causes of bleeding dysfunction facilitates the employment of evidence based, goal directed therapy to rapidly prevent and treat excess blood loss. “ Sherri Ozawa RN, Clinical Director, Institute of Patient Blood Management, Englewood Hospital Medical Center, Englewood, New Jersey. PATIENT BLOOD MANAGEMENT Interdisciplinary Managing Blood Conservation Anemia Modalities “The timely application of evidence based medical and surgical concepts designed to manage IMPROVED anemia, optimize hemostasis, and PATIENT minimize blood loss in order to OUTCOMES improve patient outcomes.” SABM© 2018 - Society for the Advancement of Blood Management (SABM.org) Optimizing Patient-Centered Coagulation Decision Making Point-of-care testing forms an integral part of PBM, enabling accurate and real-time diagnosis of anemia and bleeding and facilitating precise and targeted hemostatic intervention. 1 Conventional coagulation tests are unable to provide needed information on actual bleeding risk/defects 1, thus delaying appropriate and targeted treatment. Slow turnaround times for laboratory-based conventional coagulation tests are one of the major drivers of empirical treatment regimens, which inevitably result in some patients receiving unnecessary, inappropriate and avoidable transfusions, with associated increased morbidity and mortality, and -

Prothrombin Time (PT) Be Kept in Constant and Incubation Temperature at Step 3, Start Stop Watch , Mix in a Water Bath ( 37°C ) (LIQUID REAGENT) 36.5-37.5°C
components of any other kit with different lot numbers. temperature 15 minutes prior to testing. Throughout testing all test tubes, syringes and pipettes 2. Pre-warm PT reagent at 37°C for 5 minutes. should be plastic. 3. Pipette 100µl of PT reagent to each tube. Throughout testing all test tubes, incubation time should 4. Add 50 µl of sample, controls to the tubes prepared in Prothrombin Time (PT) be kept in constant and incubation temperature at step 3, start stop watch , mix in a water bath ( 37°C ) (LIQUID REAGENT) 36.5-37.5°C. for 8 seconds , then record the time required for clot For In-Vitro diagnostic use only If testing is delayed for more than 4 hours, plasma may formation . be stored at 2-8°C. B.Automated Method Store at: 2-8°C Each laboratory should establish a Quality Control To perform this test, refer to the appropriate Instrument program that includes both normal and abnormal control Operator’s Manual for detailed instructions. INTENDED USE plasmas to evaluate instrument, reagent tested daily Prothrombin Time (PT) is commonly used for screening for prior to performing tests on patient plasmas. Monthly RESULTS extrinsic factor deficiency, monitoring oral anticoagulant quality control charts are recommended to determine Clot time of the test Prothrombin Time plasma therapy and quantitative determination of the extrinsic the mean and standard deviation of each of the daily = Ratio (PTR) Clot time of the control coagulation factors. control plasma. All assays should include controls, and if plasma any of the controls are outside the established reference INR = PTRISI PRINCIPLE ranges, then the assay should be considered invalid and Example: for a PTR of 2.0 and an ISI of 1.0 Tissue thromboplastin, in the presence of calcium ions and no patient results should be reported. -

Global Assays of Hemostasis in the Diagnostics of Hypercoagulation and Evaluation of Thrombosis Risk Elena N Lipets1 and Fazoil I Ataullakhanov1,2,3,4,5,6*
Lipets and Ataullakhanov Thrombosis Journal (2015) 13:4 DOI 10.1186/s12959-015-0038-0 REVIEW Open Access Global assays of hemostasis in the diagnostics of hypercoagulation and evaluation of thrombosis risk Elena N Lipets1 and Fazoil I Ataullakhanov1,2,3,4,5,6* Abstract Thrombosis is a deadly malfunctioning of the hemostatic system occurring in numerous conditions and states, from surgery and pregnancy to cancer, sepsis and infarction. Despite availability of antithrombotic agents and vast clinical experience justifying their use, thrombosis is still responsible for a lion’s share of mortality and morbidity in the modern world. One of the key reasons behind this is notorious insensitivity of traditional coagulation assays to hypercoagulation and their inability to evaluate thrombotic risks; specific molecular markers are more successful but suffer from numerous disadvantages. A possible solution is proposed by use of global, or integral, assays that aim to mimic and reflect the major physiological aspects of hemostasis process in vitro. Here we review the existing evidence regarding the ability of both established and novel global assays (thrombin generation, thrombelastography, thrombodynamics, flow perfusion chambers) to evaluate thrombotic risk in specific disorders. The biochemical nature of this risk and its detectability by analysis of blood state in principle are also discussed. We conclude that existing global assays have a potential to be an important tool of hypercoagulation diagnostics. However, their lack of standardization currently impedes their application: different assays and different modifications of each assay vary in their sensitivity and specificity for each specific pathology. In addition, it remains to be seen how their sensitivity to hypercoagulation (even when they can reliably detect groups with different risk of thrombosis) can be used for clinical decisions: the risk difference between such groups is statistically significant, but not large. -

64Th Annual SSC Meeting, in Conjunction with the 2018 ISTH Congress in Dublin, Ireland Meeting Minutes Standing Committees Coagulation Standards Committee
64th Annual SSC meeting, in conjunction with the 2018 ISTH Congress in Dublin, Ireland Meeting Minutes Standing Committees Coagulation Standards Committee............................................................... 3 Subcommittees Animal, Cellular and Molecular Models ........................................................ 6 Biorheology .................................................................................................. 9 Control of Anticoagulation ............................................................................ 15 Disseminated Intravascular Coagulation ...................................................... 20 Factor VIII, Factor IX and Rare Coagulation Disorders ................................ 23 Factor XI and the Contact System ............................................................... 27 Factor XIII and Fibrinogen ............................................................................ 30 Fibrinolysis ................................................................................................... 34 Genomics in Thrombosis and Hemostasis ................................................... 38 Hemostasis and Malignancy ........................................................................ 47 Lupus Anticoagulant/Phospholipid Dependent Antibodies ........................... 51 Pediatric and Neonatal Hemostasis and Thrombosis ................................... 57 Perioperative and Critical Care Thrombosis and Hemostasis ...................... 66 Plasma Coagulation Inhibitors ..................................................................... -

Severity of Anaemia Has Corresponding Effects on Coagulation Parameters of Sickle Cell Disease Patients
diseases Article Severity of Anaemia Has Corresponding Effects on Coagulation Parameters of Sickle Cell Disease Patients Samuel Antwi-Baffour 1,* , Ransford Kyeremeh 1 and Lawrence Annison 2 1 Department of Medical Laboratory Sciences, School of Allied Health Sciences, College of Health Sciences, University of Ghana, P.O. Box KB 143 Accra, Ghana; [email protected] 2 Department of Medical Laboratory Sciences, School of Allied Health Sciences, Narh-Bita College, P.O. Box Co1061 Tema, Ghana; [email protected] * Correspondence: s.antwi-baff[email protected] Received: 31 July 2019; Accepted: 18 October 2019; Published: 17 December 2019 Abstract: Sickle cell disease (SCD) is an inherited condition characterized by chronic haemolytic anaemia. SCD is associated with moderate to severe anaemia, hypercoagulable state and inconsistent platelet count and function. However, studies have yielded conflicting results with regards to the effect of anaemia on coagulation in SCD. The purpose of this study was to determine the effect of anaemia severity on selected coagulation parameters of SCD patients. Four millilitres of venous blood samples were taken from the participants (SCD and non-SCD patients) and used for analysis of full blood count and coagulation parameters. Data was analysed using SPSS version-16. From the results, it was seen that individuals with SCD had a prolonged mean PT, APTT and high platelet count compared to the controls. There was also significant difference in the mean PT (p = 0.039), APTT (p = 0.041) and platelet count (p = 0.010) in HbSS participants with severe anaemia. Mean APTT also showed significant difference (p = 0.044) with severe anaemia in HbSC participants. -

Pre-Analytical Variables in the Coagulation Lab: Why Does It Matter?
Generate Knowledge Pre-Analytical Variables in the Coagulation Lab: Why Does It Matter? Paul Riley, PhD, MBA Pre-Analytical Variables: Objectives 1. Define Pre-Analytical variables 2. Explain how blood collection may impact test results 3. Describe best practices for sample transport and storage 4. Review sample processing procedures 5. Identify patient variables that may affect coagulation testing What are Pre-Analytical Variables? Includes everything that may affect a patient specimen from the clinician ordering the test to the point of analysis Some patient variables are out of the lab’s control (medications, lipemia, icterus, etc.) The biggest source of laboratory error - far exceeds analytical error Scope? Up to 70% of testing errors occur in the pre-analytical phase1 It is not always clear when a sample is received in the lab that it may be unsuitable or compromised Lab results lead to clinical action 70-80% of all clinical decisions regarding patient care are based on lab results Coag samples are especially susceptible Sample collection initiates clotting PT and PTT are complex enzymatic reactions 1. Plebani M: Quality indicators to detect pre-analytical errors in Laboratory testing. Clin Biochem Rev 2012; 33:85-88 Scope? What are the ramifications of pre-analytical errors for the patient? Misdiagnosis Inappropriate treatment Diligence is required on the part of the laboratory to prevent incorrect results Must have quality indicators in place to monitor the steps of the pre- analytical phase Event management where errors are investigated and corrected so that repeat events are prevented Scope? When a sample is compromised: • the test result may reflect the status of the sample • but not reflect the clinical status of the patient Guidelines In order to improve the quality of patients results, sample collection and handling should follow the CLSI guideline H21-A5; 2008 Test Ordering Is the right test ordered on the right patient? Coag is confusing - Names sound alike! Factor X vs. -

Laboratory-General Specimen Collection and Handling Guidelines
Laboratory-General Specimen Collection and Handling Guidelines Contents: Microbiology continued. Orders/Requests Stool Patient Preparation Throat or Pharynx Specimen Containers Tuberculosis (TB) Specimen Quality Urine Order of Draw Viral Specimen Transport VRE Surveillance (Vancomycin- Resistant entercoccus) Specimen Rejection Wound General Lab Sample/Source: Whole Blood Cytology (Cytopathology) Plasma Aspiration, Fine Needle Serum Aspiration, Cyst Fluids Urine Submission of slide Fecal (Stool) Tips on making smears Body Fluid Body Cavity Fluids Cerebrospinal Spinal Fluid Breast Nipple Secretions Synovial Fluid Brushing Specimens Microbiology Cerebrospinal Fluid (CSF) Sample/Source: Ectocervix, Endocervical canal, Vaginal pool Abscess (Deep aspirate) Pap Smear, Conventional Abscess (superficial swab) Pap Smear, Liquid Base Acid Fast Bacillus (AFB) Sputum Specimens Anaerobic Surface Scrape Specimen (Tzanck Smear) Aspirate, drainage, cyst fluid, or pustule Vaginal Wall (Maturation Index) Biopsy, Bone, Tissue Washing Specimens Blood (Adult) Histology (Anatomic Pathology) Blood )Pediatric Routine Submission Blood for Acid Fast Bacillus (AFB) Fresh Specimen Body Fluids Surgical Specimen and Microbiology test(s) Bronchial Washing Lavage Breast Tissue Catheter Tip Brushing Specimens C. difficile Toxin B Bronchial Washing and Brushings Chlamydia/Gonorrhea Amplified Detection Muscle Biopsy Crytococcal Antigen Renal Biopsy (Kidney) Cerebral Spinal Fluid (CSF) Renal calculi (Kidney/Bladder Stones) Ear (outer) Bone Marrow Ear (inner) Cytogenics Eye -

Point of Care Coagulation Testing
POINT OF CARE COAGULATION TESTING Dr Danny Morland Royal Victoria Infirmary Newcastle upon Tyne 11 th October 2016 Introduction Declarations of Interest: None CONTENT Introduction to POCT Principles Interpretation Treatment Literature NUTH Experience Point Of Care Testing (POCT) Medical diagnostic testing at (or near) the point of care. POCT PROS CONS • Quick • Cost (potentially) • Convenient • Quality • Reliable • Training • Efficient • Workload • Recording • Risk of inappropriate decision-making Point of Care Coagulation Testing (POCCT) Viscoelastic properties of whole blood clot Thromboelastography = Thromboelastometry (TEG) (ROTEM) Purported Benefits over Standard Tests • Measures whole blood, not just plasma • Looks at clot generation and propagation beyond the point of clot appearance • Allows comment on clot ‘quality’ • Can identify fibrinolysis FAST –potential information on clotting status within 5mins of test starting POCCT vs Standard Lab Tests POCCT LAB • Whole blood • Highly standardised • Clot beyond first • Trained, professional staff appearance • Quality control • Clot quality • Well established • Identify fibrinolysis • Complete picture • FAST • Cost PRINCIPLES Viscoelasticity Hardware OUTPUTS Panel Testing – Normal results INTERPRETATION Normal Low Platelets Normal Hypo-fibrinogenaemia Heparin Effect Normal TREATMENT LIMITATIONS AND WARNINGS • Treatment should be administered according to the clinical picture (e.g. volume & current rate of blood loss) • Viscoelastic devices are not uniformly sensitive to all disturbances -

Research Article D-Dimer and Prothrombin Time Are the Significant Indicators of Severe COVID-19 and Poor Prognosis
Hindawi BioMed Research International Volume 2020, Article ID 6159720, 10 pages https://doi.org/10.1155/2020/6159720 Research Article D-Dimer and Prothrombin Time Are the Significant Indicators of Severe COVID-19 and Poor Prognosis Hui Long,1 Lan Nie,2 Xiaochen Xiang,2 Huan Li,1 Xiaoli Zhang,1 Xiaozhi Fu,1 Hongwei Ren,1 Wanxin Liu,2 Qiang Wang ,2 and Qingming Wu 1,2 1Internal Medicine of Tianyou Hospital, Wuhan University of Science and Technology, Wuhan 430064, China 2Institute of Infection, Immunology and Tumor Microenvironment, Hubei Province Key Laboratory of Occupational Hazard Identification and Control, Medical College, Wuhan University of Science and Technology, Wuhan 430065, China Correspondence should be addressed to Qiang Wang; [email protected] and Qingming Wu; [email protected] Received 22 April 2020; Accepted 19 May 2020; Published 17 June 2020 Academic Editor: Frederick D. Quinn Copyright © 2020 Hui Long et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. Objective. To investigate the value of coagulation indicators D-dimer (DD), prothrombin time (PT), activated partial thromboplastin time (APTT), thrombin time (TT), and fibrinogen (Fg) in predicting the severity and prognosis of COVID-19. Methods. A total of 115 patients with confirmed COVID-19, who were admitted to Tianyou Hospital of Wuhan University of Science and Technology between January 18, 2020, and March 5, 2020, were included. The dynamic changes of DD, PT, APTT, and Fg were tested, and the correlation with CT imaging, clinical classifications, and prognosis was studied. -

Thrombophilia Testing Panel Now Available
Thrombophilia Testing Panel Now Available Bronson Laboratory offers a any impact on the patient’s therapy. Importantly, most people with an thrombophilia testing panel consisting For instance, the vast majority of older inheritable thrombophilic mutation will of the following: factor VIII activity, anti- patients with venous thromboses in the never experience a thrombotic event, thrombin III activity, protein setting of a recognized inciting event and most of those who do will never C activity, protein S antigen, factor V Leiden will not have a preexisting thrombophilic do so again. Because of this, screening mutation assay, prothrombin 90210A condition, and accordingly testing is the general population or even asympto- mutation assay, and a lupus anticoagulant generally not recommended in this pop- matic relatives of individuals with thrombo- assay. Abnormal values for protein C, ulation. In regards to arterial thromboses, philic mutations is discouraged because protein S, and antithrombin III will be the overwhelming majority are a direct of the potential for harmful overtreatment. automatically reflexed to confirmatory consequence of atherosclerosis, and the By similar reasoning, some hematologists testing at the Mayo Clinic laboratories. added presence of a thrombophilia in would recommend the same period of Although these tests are orderable indi- this setting will not affect patient anticoagulation, often 3-6 months after vidually, there is little rationale to do so management. (continued on page 4) except as noted below. Clinical circumstances that suggest The activity assays are clotting tests a laboratory evaluation for thrombophilia that require adequate amounts of func- tional protein to achieve a normal result. For reasons having to do with greater achievable precision, the free protein S Patient with a Asymptomatic patients Other clinical situations with a family history of VTE antigen test is used in lieu of an activity confirmed acute VTE assay, but it too depends on adequate amounts of circulating protein for a normal finding.