Cannabis Testing Standards
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Targeting the Endocannabinoid System to Reduce Nociception
Virginia Commonwealth University VCU Scholars Compass Theses and Dissertations Graduate School 2011 Targeting the endocannabinoid system to reduce nociception Lamont Booker Virginia Commonwealth University Follow this and additional works at: https://scholarscompass.vcu.edu/etd Part of the Medical Pharmacology Commons © The Author Downloaded from https://scholarscompass.vcu.edu/etd/2419 This Dissertation is brought to you for free and open access by the Graduate School at VCU Scholars Compass. It has been accepted for inclusion in Theses and Dissertations by an authorized administrator of VCU Scholars Compass. For more information, please contact [email protected]. Targeting the Endocannabinoid System to Reduce Nociception A dissertation submitted in partial fulfillment of the requirements for the degree of Doctor of Philosophy at Virginia Commonwealth University. By Lamont Booker Bachelor’s of Science, Fayetteville State University 2003 Master’s of Toxicology, North Carolina State University 2005 Director: Dr. Aron H. Lichtman, Professor, Pharmacology & Toxicology Virginia Commonwealth University Richmond, Virginia April 2011 Acknowledgements The author wishes to thank several people. I like to thank my advisor Dr. Aron Lichtman for taking a chance and allowing me to work under his guidance. He has been a great influence not only with project and research direction, but as an excellent example of what a mentor should be (always willing to listen, understanding the needs of each student/technician, and willing to provide a hand when available). Additionally, I like to thank all of my committee members (Drs. Galya Abdrakmanova, Francine Cabral, Sandra Welch, Mike Grotewiel) for your patience and willingness to participate as a member. Our term together has truly been memorable! I owe a special thanks to Sheryol Cox, and Dr. -

Cannabinoid As Potential Aromatase Inhibitor Through Molecular Modeling and Screening for Anti-Cancer Activity
Cannabinoid as Potential Aromatase Inhibitor Through Molecular Modeling and Screening for Anti-Cancer Activity Sudipta Baroi1, Achintya Saha2, Ritesh Bachar3 and Sitesh C Bachar4 1Department of Pharmaceutical Technology, Faculty of Pharmacy, University of Dhaka Dhaka-1000, Bangladesh 2Department of Chemical Technology, Pharmaceutical & Fine Chemical Technology Division University of Calcutta, India 3Department of Pharmacy, School of Science and Engineering, University of Information Technology and Sciences (UITS), Dhaka-1212, Bangladesh 4Department of Pharmacy, Faculty of Pharmacy, University of Dhaka, Dhaka-1000, Bangladesh (Received: April 15, 2020; Accepted: June 2, 2020; Published (web): June 28, 2020) ABSTRACT: Inhibition of aromatase (CYTP450), a key enzyme in the estrogen biosynthesis, could result in regression of estrogen-dependent tumors and even prevent the promotion of breast cancer. The present research has been designed for searching a potent chemical moiety from natural sources to inhibit aromatase enzyme, the over- functionality of which causes the breast cancer. Cannabis sativa contains a very much promising group of cannabinoids with more than 66 compounds with reported anticancer property and for the search of a target specific potent aromatase inhibitor, 61 cannabinoids from C. sativa were selected. The Structures Data File (SDF) of these ligand molecules were subjected to docking studies at the binding site of aromatase X-ray crystallographic structure based on lower resolution of the protein crystal structure and higher docking accuracy, predicted by calculating the correlation between experimental activities and Glide dock scores and compared with the standard aromatase ligand androstenedione and aromatase inhibitor fadrozole with existing drug for breast cancer treatment. The best docked pose of each ligand was selected on the basis of the highest dock score related to the binding free energies of the internal dataset compounds as compared to their observed activities. -
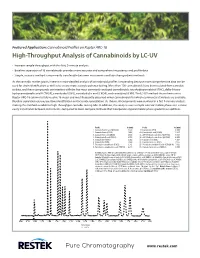
High-Throughput Analysis of Cannabinoids by LC-UV
Featured Application: Cannabinoid Profiles on Raptor ARC-18 High-Throughput Analysis of Cannabinoids by LC-UV • Increase sample throughput with this fast, 9-minute analysis. • Baseline separation of 16 cannabinoids provides more accurate and comprehensive potency and profile data. • Simple, isocratic method is more easily transferable between instruments and labs than gradient methods. As the cannabis market grows, interest in more detailed analysis of cannabinoid profiles is expanding because more comprehensive data can be used for strain identification as well as to ensure more accurate potency testing. More than 100 cannabinoids have been isolated from cannabis to date, and these compounds can interfere with the five most commonly analyzed cannabinoids: tetrahydrocannabinol (THC), delta-9-tetra- hydrocannabinolic acid A (THCA), cannabidiol (CBD), cannabidiolic acid (CBDA), and cannabinol (CBN). The LC-UV method shown here uses a Raptor ARC-18 column to fully resolve 16 major and most frequently observed minor cannabinoids for which commercial standards are available. Baseline separation ensures positive identification and accurate quantitation. As shown, all compounds were resolved in a fast 9-minute analysis, making this method suitable for high-throughput cannabis testing labs. In addition, this analysis uses a simple isocratic mobile phase so it is more easily transferable between instruments, compared to more complex methods that incorporate atypical mobile phase gradients or additives. Peaks tR (min) Peaks tR (min) 1. Cannabidivarinic acid (CBDVA) 1.877 9. Cannabinol (CBN) 4.609 2. Cannabidivarin (CBDV) 2.086 10. Cannabinolic acid (CBNA) 5.437 3. Cannabidiolic acid (CBDA) 2.592 11. Δ9-Tetrahydrocannabinol (Δ9-THC) 5.815 4. Cannabigerolic acid (CBGA) 2.750 12. -

CBD (Cannabidiol)
TRANSPORTATION RESEARCH BOARD Driving Toward the Truth - Dispelling the Myths About Cannabis Products February 10, 2021 @NASEMTRB #TRBwebinar PDH Certification The Transportation Research Board has met the standards and Information: requirements of the Registered Continuing Education Providers •1.5 Professional Development Program. Credit earned on completion Hour (PDH) – see follow-up of this program will be reported to email for instructions RCEP. A certificate of completion will •You must attend the entire be issued to participants that have registered and attended the entire webinar to be eligible to receive session. As such, it does not include PDH credits content that may be deemed or •Questions? Contact Reggie construed to be an approval or Gillum at [email protected] endorsement by RCEP. #TRBwebinar Learning Objectives 1. Identify impacts of the Farm Bill on use of THC and CBD products 2. Describe the toxicology of THC and CBD products 3. Discuss how THC and CBD products affect driving performance and crash risk #TRBwebinar TRB Standing Committee on Impairment in Transportation (ACS50) TRB Webinar: Driving Toward the Truth - Dispelling the Myths About Cannabis Products Dr. Barry K. Logan Executive Director, Center for Forensic Science Research and Education (CFSRE); Senior Vice President of Forensic Sciences, and Chief Scientist at NMS Labs Michelle Peace, Ph.D. Associate Professor and PI, Laboratory for Forensic Toxicology Research Department of Forensic Science, Virginia Commonwealth University Dr. Darrin Grondel Vice President, -

Potential Health Benefits of Cannabis Extracts: a Review Maria Rosana Ramirez
International Journal of Chemical and Biomedical Science 2016; 2(1): 1-8 Published online March 1, 2016 (http://www.aascit.org/journal/ijcbs) Potential Health Benefits of Cannabis Extracts: A Review Maria Rosana Ramirez CITER-The National Scientific and Technical Research Council (CONICET), Argentine Government, Agency, Argentina Email address [email protected] Citation Maria Rosana Ramirez. Potential Health Benefits of Cannabis Extracts: A Review. International Journal of Chemical and Biomedical Science . Vol. 2, No. 1, 2016, pp. 1-8. Keywords Abstract Non-cannabinoids Compounds, A central tenet underlying the use of plant preparations is that herbs contain many Cannabis Extracts, bioactive compounds. Cannabis contains tetrahydrocannabinols (THC) a primary Bioactivity metabolite with reported psychotropic effects. Therefore, the presence of THC makes controversial the use of Cannabis to treat diseases by which their uses and applications were limited. The question then is: is it possible to use the extracts from Cannabis to treat the diseases related with it use in folk medicine? More recently, the synergistic Received: January 24, 2016 contributions of bioactive constituents have been scientifically demonstrated. We Revised: February 10, 2016 reviewed the literature concerning medical cannabis and its secondary metabolites, Accepted: February 12, 2016 including fraction and total extracts. Scientific evidence shows that secondary metabolites in cannabis may enhance the positive effects of THC a primary metabolite. Other chemical components (cannabinoid and non-cannabinoid) in cannabis or its extracts may reduce THC-induced anxiety, cholinergic deficits, and immunosuppression; which could increase its therapeutic potential. Particular attention will be placed on non- cannabinoid compounds interactions that could produce synergy with respect to treatment of pain, inflammation, epilepsy, fungal and bacterial infections. -

Designing Microorganisms for Heterologous Biosynthesis of Cannabinoids Angelaˆ Carvalho1, Esben Halkjær Hansen1, Oliver Kayser2, Simon Carlsen1,∗ and Felix Stehle2
FEMS Yeast Research, 17, 2017, fox037 doi: 10.1093/femsyr/fox037 Advance Access Publication Date: 4 June 2017 Minireview MINIREVIEW Designing microorganisms for heterologous biosynthesis of cannabinoids Angelaˆ Carvalho1, Esben Halkjær Hansen1, Oliver Kayser2, Simon Carlsen1,∗ and Felix Stehle2 1Evolva Biotech A/S, Lersø Parkalle´ 42-44, 2100, Copenhagen, Denmark and 2Laboratory of Technical Biochemistry, Department of Biochemical and Chemical Engineering, TU Dortmund University, Emil-Figge-Str. 66, 44227 Dortmund, Germany ∗Corresponding author: Evolva Biotech A/S, Lersø Parkalle´ 42-44, 2100, Copenhagen, Denmark. Tel: +45-35-200-243; E-mail: [email protected] One sentence summary: In this review, the authors explore the use of synthetic biology as an alternative approach for the synthesis of pharmaceutical cannabinoids in a heterologous host organism. Editor: John Morrissey ABSTRACT During the last decade, the use of medical Cannabis has expanded globally and legislation is getting more liberal in many countries, facilitating the research on cannabinoids. The unique interaction of cannabinoids with the human endocannabinoid system makes these compounds an interesting target to be studied as therapeutic agents for the treatment of several medical conditions. However, currently there are important limitations in the study, production and use of cannabinoids as pharmaceutical drugs. Besides the main constituent tetrahydrocannabinolic acid, the structurally related compound cannabidiol is of high interest as drug candidate. From the more than 100 known cannabinoids reported, most can only be extracted in very low amounts and their pharmacological profile has not been determined. Today, cannabinoids are isolated from the strictly regulated Cannabis plant, and the supply of compounds with sufficient quality is a major problem. -

Recent Developments in Cannabis Chemistry
Recent Developments in Cannabis Chemistry BY ALEXANDER T. SHULGIN, Ph.D. The marijuana plant Cannabis sativa contains a bewildering Introduction array of organic chemicals. As is true with other botanic species, there are representatives of almost all chemical classes present, including mono- and sesquiterpenes, carbohy- drates, aromatics, and a variety of nitrogenous compounds. Interest in the study of this plant has centered primarily on the resinous fraction, as it is this material that is invested with the pharmacological activity that is peculiar to the plant. This resin is secreted by the female plant as a protective agent during seed ripening, although it can be found as a microscopic exudate through the aerial portions of plants of either sex. The pure resin, hashish or charas, is the most potent fraction of the plant, and has served as the source material for most of the chemical studies. The family of chemicals that has been isolated from this source has been referred to as the cannabinoid group. It is unique amongst psychotropic materials from plants in that there are no alkaloids present. The fraction is totally nitro- gen-free. Rather, the set of compounds can be considered as analogs of the parent compound cannabinol (I), a fusion product of terpene and a substituted resorcinol. Beyond the scope of this present review are such questions as the distribution of these compounds within the plant, the bo- tanic variability resulting from geographic distribution, the diversity of pharmacological action assignable to the several Reprinted from Journal of Psychedelic Drugs, vol. II, no. 1, 197 1. 397 398 Marijuana: Medical Papers distinct compounds present, and the various preparations and customs of administration. -

What Is Delta-8 THC?? Cannabinoid Chemistry 101
What is Delta-8 THC?? Cannabinoid Chemistry 101 National Conference on Weights and Measures Annual Meeting - Rochester, NY Matthew D. Curran, Ph.D. July 21, 2021 Disclaimer Just to be clear… • I am a chemist and not a lawyer so: • This presentation will not discuss the legal aspects of Δ8-THC or DEA’s current position. • This presentation will not discuss whether Δ8-THC is considered “synthetic” or “naturally occurring.” • This is not a position statement on any issues before the NCWM. • Lastly, this should only be considered a scientific sharing exercise. Florida Department of Agriculture and Consumer Services 2 Cannabis in Florida Cannabis Syllabus • What is Cannabis? • “Mother” Cannabinoid • Decarboxylation • Relationship between CBD and THC • What does “Total” mean? • Dry Weight vs. Wet Weight • What does “Delta-9” mean? • Relationship between “Delta-8” and “Delta-9” • CBD to Delta-8 THC • Cannabinoid Chemistry 202… Florida Department of Agriculture and Consumer Services 3 Cannabis Cannabis • Cannabis sativa is the taxonomic name for the plant. • The concentration of Total Δ9-Tetrahydrocannabinol (Total Δ9-THC) is critical when considering the varieties of Cannabis sativa. • Hemp – (Total Δ9-THC) 0.3% or less • Not really a controversial term, “hemp” • Marijuana/cannabis – (Total Δ9-THC) Greater than 0.3% • Controversial term, “marijuana” • Some states prohibit the use of this term whereas some states have it in their laws. • Some states use the term “cannabis.” • Not italicized • Lower case “c” Florida Department of Agriculture and -

Roles of Cannabidiol in Reversing Proteinopathies
Preprints (www.preprints.org) | NOT PEER-REVIEWED | Posted: 21 May 2020 doi:10.20944/preprints202005.0340.v1 Review Roles of Cannabidiol in Reversing Proteinopathies Raju Dash1, Md. Chayan Ali2†, Israt Jahan3†, Yeasmin Akter Munni1†, Sarmistha Mitra4, Md. Abdul Hannan1,5, Binod Timalsina1, Diyah Fatimah Oktaviani1, Ho Jin Choi1, Il Soo Moon1* 1Department of Anatomy, Dongguk University College of Medicine, Gyeongju 38066, Republic of Korea 2Department of Biotechnology and Genetic Engineering, Faculty of Biological Sciences, Islamic University, Kushtia 7003, Bangladesh 3Department of Pharmacy, Faculty of Life and Earth Sciences, Jagannath University, Dhaka 1100, Bangladesh. 4Plasma Bioscience Research Center and Department of Plasma Bio display, Kwangwoon University, Seoul 01897, Republic of Korea 5Department of Biochemistry and Molecular Biology, Bangladesh Agricultural University, Mymensingh 2202, Bangladesh †These authors have contributed equally. *Corresponding author: Il Soo Moon Mailing address: Department of Anatomy, Dongguk University College of Medicine, 123 Dongdae-ro, Gyeongju 38066, Republic of Korea Phone: +82-54-770-2414 Fax: +82-54-770-2447 Email: [email protected] Abstract: Cannabidiol is a well-known non-psychotropic phytocannabinoid from Cannabis sativa, which exerts a broad range of neuropharmacological activities in the central nervous systems. Over the past years, compelling evidence from preclinical and clinical studies support therapeutic potentials of cannabidiol in various neurological disorders, including neurodegenerative diseases. Neurodegenerative diseases are characterized by the accumulation of misfolded or aggregated protein due to the defective protein homeostasis or proteostasis network, termed as proteinopathies. Because of its role in the protein homeostasis network, cannabidiol could be a potent molecule to revert not only age-associated neurodegeneration but also other protein misfolding disorders. -

In Silico Assessment of Drug-Like Properties of Phytocannabinoids in Cannabis Sativa
EDUCATUM JSMT Vol. 4 No. 2 (2017) ISSN 2289-7070 / eISSN 2462-2451 (1-7) https://ejournal.upsi.edu.my/journal/EDSC In Silico Assessment of Drug-Like Properties of Phytocannabinoids in Cannabis Sativa Shakinaz Desa1*, Asiah Osman2, and Richard Hyslop3 1Department of Biology, Universiti Pendidikan Sultan Idris, Malaysia, 2Natural Product Division, Forest Research Institute Malaysia, 3Department of Chemistry and Biochemistry, University of Northern Colorado, USA *Corresponding author: [email protected] Abstract This study investigated drug-like properties of phytocannabinoids in Cannabis sativa using an in silico study. We report sixteen phytocannabinoids: cannabidiol (CBD), cannabidiolic acid (CBDA), cannabinol (CBN), cannabichromene (CBC), cannabigerol (CBG), cannabicyclol (CBL), cannabivarin (CBV), cannabidivarin (CBDV), cannabichromevarin (CBCV), cannabigerovarin (CBGV), cannabinodiol (CBDL), cannabielsoin (CBE), cannabitriol (CBT), Δ9-tetrahydrocannabinol (Δ9-THC), Δ9-tetrahydrocannabivarin (Δ9-THCV), and Δ8-tetrahydrocannabinol (Δ8-THC). All chemical structures and properties were obtained from PubChem Compound, National Center for Biotechnology Information, U.S. National Library of Medicine. Molinspiration was used for the calculation of molecular properties and bioactivity score. The parameters were molecular weight (MW), number of hydrogen acceptor (HBA), number of hydrogen donor (HBD), partition coefficient (cLogP), polar surface area (PSA) and number of rotatable bonds (NROTB). We predicted bioactivity scores for G Protein-Coupled Receptors (GPCR) ligand, ion channel modulator, kinase inhibitor, nuclear receptor ligand, protease inhibitor and enzyme inhibitor. Lipinski’s rule was used as reference to determine the drug-like properties of the phytocannabinoids. All compounds have MW<500, HBA<10, HBD<5, TPSA<140Å2 and NRTOB<10. Bioactivity score showed an active or moderately active in all compounds. -

The Handbook of Cannabis Therapeutics: from Bench to Bedside
Handbook of Cannabis Therapeutics From Bench to Bedside 9780789030979 Handbook of Cannabis Therapeutics From Bench to Bedside Size: 212 x 152mm Spine size: 26 mm Color pages: Binding: Paperback THE HAWORTH PRESS® Haworth Series in Integrative Healing Ethan Russo Editor The Last Sorcerer: Echoes of the Rainforest by Ethan Russo Professionalism and Ethics in Complementary and Alternative Medicine by John Crellin and Fernando Ania Cannabis and Cannabinoids: Pharmacology, Toxicology, and Therapeutic Potential by Franjo Grotenhermen and Ethan Russo Modern Psychology and Ancient Wisdom: Psychological Healing Practices from the World’s Religious Traditions edited by Sharon G. Mijares Complementary and Alternative Medicine: Clinic Design by Robert A. Roush Herbal Voices: American Herbalism Through the Words of American Herbalists by Anne K. Dougherty The Healing Power of Chinese Herbs and Medicinal Recipes by Joseph P. Hou and Youyu Jin Alternative Therapies in the Treatment of Brain Injury and Neurobehavioral Disorders: A Practical Guide edited by Gregory J. Murrey Handbook of Cannabis Therapeutics: From Bench to Bedside edited by Ethan B. Russo and Franjo Grotenhermen Handbook of Cannabis Therapeutics From Bench to Bedside Ethan B. Russo, MD Franjo Grotenhermen, MD Editors Routledge Taylor &. Francis Croup NEW YORK AND LONDON First Published by The Haworth Press, Inc., 10 Alice Street, Binghamton, NY 13904-1580. Transferred to Digital Printing 2010 by Routledge 270 Madison Ave, New York NY 10016 2 Park Square, Milton Park, Abingdon, Oxon, OX14 4RN For more information on this book or to order, visit http://www.haworthpress.com/store/product.asp?sku=5741 or call 1-800-HAWORTH (800-429-6784) in the United States and Canada or (607) 722-5857 outside the United States and Canada or contact [email protected] © 2006 by The Haworth Press, Inc. -

Preparative Isolation of Cannabinoids from Cannabis Sativa by Centrifugal Partition Chromatography
See discussions, stats, and author profiles for this publication at: https://www.researchgate.net/publication/244594577 Preparative Isolation of Cannabinoids from Cannabis sativa by Centrifugal Partition Chromatography Article in Journal of Liquid Chromatography & Related Technologies · December 2004 DOI: 10.1081/JLC-200028170 CITATIONS READS 47 2,645 5 authors, including: Arno Hazekamp Robert Verpoorte Bedrocan BV Leiden University 50 PUBLICATIONS 975 CITATIONS 732 PUBLICATIONS 21,203 CITATIONS SEE PROFILE SEE PROFILE Some of the authors of this publication are also working on these related projects: The effects of inhaled delta-9-tetrahydrocannabinol (THC) and cannabidiol (CBD) on pain relief and subjective effects in fibromyalgia patients View project All content following this page was uploaded by Robert Verpoorte on 20 June 2014. The user has requested enhancement of the downloaded file. Cannabis; extracting the medicine i Arno Hazekamp Cannabis; extracting the medicine Proefschrift Universiteit Leiden ISBN 978-90-9021997-4 Printed by PrintPartners Ipskamp B.V., Amsterdam, The Netherlands Paper cover: Cannabis Pur 100% (250 grams) from Grafisch Papier, The Nederlands. Photo cover: Dutch medicinal cannabis, variety “Bedrocan”. ii Cannabis; extracting the medicine Proefschrift Ter verkrijging van de graad van Doctor aan de Universiteit Leiden, op gezag van de Rector Magnificus prof. mr. P. F. van der Heijden, hoogleraar in de faculteit der Rechtsgeleerdheid, volgens besluit van het College voor Promoties te verdedigen op woensdag 5 september 2007 klokke 15.00 uur door Arno Hazekamp Geboren op 15 maart 1976 te Bilthoven iii Promotiecommissie Promotor Prof. dr. R. Verpoorte Referent Dr. C. Giroud (Institut Universitaire de Médecine Légale, Lausanne, Switzerland) Overige leden Prof.