Searching for Diamonds in the Apomictic Rough: a Case Study Involving Boechera Lignifera (Brassicaceae)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Chromosomal Evolution and Apomixis in the Cruciferous Tribe Boechereae
fpls-11-00514 May 26, 2020 Time: 17:57 # 1 ORIGINAL RESEARCH published: 28 May 2020 doi: 10.3389/fpls.2020.00514 Chromosomal Evolution and Apomixis in the Cruciferous Tribe Boechereae Terezie Mandáková1, Petra Hloušková1, Michael D. Windham2, Thomas Mitchell-Olds2, Kaylynn Ashby3, Bo Price3, John Carman3 and Martin A. Lysak1* 1 CEITEC, Masaryk University, Brno, Czechia, 2 Department of Biology, Duke University, Durham, NC, United States, 3 Plants, Soils, and Climate Department, Utah State University, Logan, UT, United States The mustard family (Brassicaceae) comprises several dozen monophyletic clades usually ranked as tribes. The tribe Boechereae plays a prominent role in plant research due to the incidence of apomixis and its close relationship to Arabidopsis. This tribe, largely confined to western North America, harbors nine genera and c. 130 species, with >90% of species belonging to the genus Boechera. Hundreds of apomictic diploid and triploid Boechera hybrids have spurred interest in this genus, but the remaining Boechereae genomes remain virtually unstudied. Here we report on comparative Edited by: genome structure of six genera (Borodinia, Cusickiella, Phoenicaulis, Polyctenium, Steven Dodsworth, Nevada, and Sandbergia) and three Boechera species as revealed by comparative University of Bedfordshire, United Kingdom chromosome painting (CCP). All analyzed taxa shared the same seven-chromosome Reviewed by: genome structure. Comparisons with the sister Halimolobeae tribe (n = 8) showed Ana Paula Moraes, that the ancestral Boechereae genome (n = 7) was derived from an older n = 8 Universidade Federal do ABC, Brazil Aretuza Sousa Dos Santos, genome by descending dysploidy followed by the divergence of extant Boechereae Ludwig Maximilian University taxa. -

Taxa Named in Honor of Ihsan A. Al-Shehbaz
TAXA NAMED IN HONOR OF IHSAN A. AL-SHEHBAZ 1. Tribe Shehbazieae D. A. German, Turczaninowia 17(4): 22. 2014. 2. Shehbazia D. A. German, Turczaninowia 17(4): 20. 2014. 3. Shehbazia tibetica (Maxim.) D. A. German, Turczaninowia 17(4): 20. 2014. 4. Astragalus shehbazii Zarre & Podlech, Feddes Repert. 116: 70. 2005. 5. Bornmuellerantha alshehbaziana Dönmez & Mutlu, Novon 20: 265. 2010. 6. Centaurea shahbazii Ranjbar & Negaresh, Edinb. J. Bot. 71: 1. 2014. 7. Draba alshehbazii Klimeš & D. A. German, Bot. J. Linn. Soc. 158: 750. 2008. 8. Ferula shehbaziana S. A. Ahmad, Harvard Pap. Bot. 18: 99. 2013. 9. Matthiola shehbazii Ranjbar & Karami, Nordic J. Bot. doi: 10.1111/j.1756-1051.2013.00326.x, 10. Plocama alshehbazii F. O. Khass., D. Khamr., U. Khuzh. & Achilova, Stapfia 101: 25. 2014. 11. Alshehbazia Salariato & Zuloaga, Kew Bulletin …….. 2015 12. Alshehbzia hauthalii (Gilg & Muschl.) Salariato & Zuloaga 13. Ihsanalshehbazia Tahir Ali & Thines, Taxon 65: 93. 2016. 14. Ihsanalshehbazia granatensis (Boiss. & Reuter) Tahir Ali & Thines, Taxon 65. 93. 2016. 15. Aubrieta alshehbazii Dönmez, Uǧurlu & M.A.Koch, Phytotaxa 299. 104. 2017. 16. Silene shehbazii S.A.Ahmad, Novon 25: 131. 2017. PUBLICATIONS OF IHSAN A. AL-SHEHBAZ 1973 1. Al-Shehbaz, I. A. 1973. The biosystematics of the genus Thelypodium (Cruciferae). Contrib. Gray Herb. 204: 3-148. 1977 2. Al-Shehbaz, I. A. 1977. Protogyny, Cruciferae. Syst. Bot. 2: 327-333. 3. A. R. Al-Mayah & I. A. Al-Shehbaz. 1977. Chromosome numbers for some Leguminosae from Iraq. Bot. Notiser 130: 437-440. 1978 4. Al-Shehbaz, I. A. 1978. Chromosome number reports, certain Cruciferae from Iraq. -

COLLECTION SPECIES from POTENTILLA GENUS Romanian
NATURAL RESOURCES AND SUSTAINABLE DEVELOPMENT, _ 2017 COLLECTION SPECIES FROM POTENTILLA GENUS Crișan Vlad*, Dincă Lucian*, Onet Cristian**, Onet Aurelia** *National Institute for Research and Development in Forestry (INCDS) „Marin Dracea”, 13 Cloșca St., 500040, Brașov, Romania, e-mail: [email protected] **University of Oradea, Faculty of Environmental Protection, 26 Gen. Magheru St., 410048, Oradea, Romania Abstract The present paper reunites the morphological and ecological description of the main species belonging to Potentilla genus present in "Alexandru Beldie" Herbarium from Romanian National Institute for Research and Development in Forestry "Marin Drăcea" (INCDS), Bucharest. Furthermore, the paper systemize the herbarium specimens based on species, harvest year, the place from where they were harvested and the specialist that gathered them. The first part of the article shortly describes the herbarium and its specific, together with a presentation of the material and method used for elaborating this paper. As such, the material that was used is represented by the 276 plates that contain the specimens of 69 species belonging to the Potentilla genus. Besides the description of harvested Potentilla species, the article presents the European map of their harvesting locations, together with a synthetic analysis of their harvesting periods. The paper ends with a series of conclusions regarding the analysis of the Potentilla genus species and specimens present in the herbarium. Key words: herbar, plante, flowers, frunze, Potentilla. INTRODUCTION Romanian National Institute for Research and Development in Forestry "Marin Drăcea" (INCDS) from Bucharest hosts an extremely valuable collection of herbaceous plants. This herbarium is registered in "INDEX HERBARIORUM" which is a guide to the world's herbaria and their staff established since 1935. -

Diploid Apomicts of the Boechera Holboellii Complex Display Large-Scale Chromosome Substitutions and Aberrant Chromosomes
Diploid apomicts of the Boechera holboellii complex display large-scale chromosome substitutions and aberrant chromosomes Laksana Kantama*†, Timothy F. Sharbel‡, M. Eric Schranz§, Thomas Mitchell-Olds¶, Sacco de Vries*, and Hans de Jongʈ** *Laboratory of Biochemistry, Wageningen University, Dreijenlaan 3, NL-6703 HA, Wageningen, The Netherlands; ‡Apomixis Research Group, Department of Cytogenetics and Genome Analysis, Leibniz Institute of Plant Genetics and Crop Plant Research, D-06466 Gatersleben, Germany; §Institute for Biodiversity and Ecosystem Dynamics, University of Amsterdam, Kruislaan 318, NL-1098 MS, Amsterdam, The Netherlands; ¶Department of Biology, Duke University, Durham, NC 27708; and ʈLaboratory of Genetics, Wageningen University, Arboretumlaan 4, NL-6703 BD, Wageningen, The Netherlands Communicated by Maarten Koornneef, Wageningen University and Research Centre, Wageningen, The Netherlands, July 15, 2007 (received for review May 20, 2007) We conducted a cytogenetic study of sexual lines of Boechera holboellii is polyphyletic. Its sequence and microsatellite analyses and seven diploid apomic- have shown that B. divaricarpa arose through hybridization (14 ؍ stricta and Boechera holboellii (2n tic accessions of their interspecific hybrid Boechera divaricarpa and between sexual B. stricta and B. holboellii or a closely related or 15). By studying chromosome morphology, species (3, 5, 6). The level of allelic variation is comparable 14 ؍ B. holboellii (2n rDNA repeats, genome painting, male meiosis, pollen morphology, between B. divaricarpa and B. holboellii, and a low number of and flow-cytometry seed screens, we revealed an unexpected species-specific alleles suggests that the hybrid originated re- plethora of chromosome forms, pairing behavior, and hybrid cently (6). Multiple evolutionary origins of triploidy in Boechera composition in all apomictic lines. -

Evolution and Diversification of the Plant Gibberellin Receptor GID1
Evolution and diversification of the plant gibberellin receptor GID1 Hideki Yoshidaa,b, Eiichi Tanimotoc, Takaaki Hiraia, Yohei Miyanoirid,e, Rie Mitania, Mayuko Kawamuraa, Mitsuhiro Takedad,f, Sayaka Takeharaa, Ko Hiranoa, Masatsune Kainoshod,g, Takashi Akagih, Makoto Matsuokaa,1, and Miyako Ueguchi-Tanakaa,1 aBioscience and Biotechnology Center, Nagoya University, Nagoya, 464-8601 Aichi, Japan; bKihara Institute for Biological Research, Yokohama City University, Yokohama, 244-0813 Kanagawa, Japan; cGraduate School of Natural Sciences, Nagoya City University, Nagoya, 467-8501 Aichi, Japan; dStructural Biology Research Center, Graduate School of Science, Nagoya University, Nagoya, 464-8601 Aichi, Japan; eResearch Center for State-of-the-Art Functional Protein Analysis, Institute for Protein Research, Osaka University, Suita, 565-0871 Osaka, Japan; fDepartment of Structural BioImaging, Faculty of Life Sciences, Kumamoto University, 862-0973 Kumamoto, Japan; gGraduate School of Science and Engineering, Tokyo Metropolitan University, Hachioji, 192-0397 Tokyo, Japan; and hGraduate School of Agriculture, Kyoto University, 606-8502 Kyoto, Japan Edited by Mark Estelle, University of California, San Diego, La Jolla, CA, and approved July 10, 2018 (received for review April 9, 2018) The plant gibberellin (GA) receptor GID1 shows sequence similarity erwort Marchantia polymorpha (5–7). Furthermore, Hirano et al. to carboxylesterase (CXE). Here, we report the molecular evolution (5) reported that GID1s in the lycophyte Selaginella moellen- of GID1 from establishment to functionally diverse forms in dorffii (SmGID1s) have unique properties in comparison with eudicots. By introducing 18 mutagenized rice GID1s into a rice angiosperm GID1s: namely, lower affinity to bioactive GAs and gid1 null mutant, we identified the amino acids crucial for higher affinity to inactive GAs (lower specificity). -

Phylogenetic Position and Generic Limits of Arabidopsis (Brassicaceae)
PHYLOGENETIC POSITION Steve L. O'Kane, Jr.2 and Ihsan A. 3 AND GENERIC LIMITS OF Al-Shehbaz ARABIDOPSIS (BRASSICACEAE) BASED ON SEQUENCES OF NUCLEAR RIBOSOMAL DNA1 ABSTRACT The primary goals of this study were to assess the generic limits and monophyly of Arabidopsis and to investigate its relationships to related taxa in the family Brassicaceae. Sequences of the internal transcribed spacer region (ITS-1 and ITS-2) of nuclear ribosomal DNA, including 5.8S rDNA, were used in maximum parsimony analyses to construct phylogenetic trees. An attempt was made to include all species currently or recently included in Arabidopsis, as well as species suggested to be close relatives. Our ®ndings show that Arabidopsis, as traditionally recognized, is polyphyletic. The genus, as recircumscribed based on our results, (1) now includes species previously placed in Cardaminopsis and Hylandra as well as three species of Arabis and (2) excludes species now placed in Crucihimalaya, Beringia, Olimar- abidopsis, Pseudoarabidopsis, and Ianhedgea. Key words: Arabidopsis, Arabis, Beringia, Brassicaceae, Crucihimalaya, ITS phylogeny, Olimarabidopsis, Pseudoar- abidopsis. Arabidopsis thaliana (L.) Heynh. was ®rst rec- netic studies and has played a major role in un- ommended as a model plant for experimental ge- derstanding the various biological processes in netics over a half century ago (Laibach, 1943). In higher plants (see references in Somerville & Mey- recent years, many biologists worldwide have fo- erowitz, 2002). The intraspeci®c phylogeny of A. cused their research on this plant. As indicated by thaliana has been examined by Vander Zwan et al. Patrusky (1991), the widespread acceptance of A. (2000). Despite the acceptance of A. -

Biogeography and Diversification of Brassicales
Molecular Phylogenetics and Evolution 99 (2016) 204–224 Contents lists available at ScienceDirect Molecular Phylogenetics and Evolution journal homepage: www.elsevier.com/locate/ympev Biogeography and diversification of Brassicales: A 103 million year tale ⇑ Warren M. Cardinal-McTeague a,1, Kenneth J. Sytsma b, Jocelyn C. Hall a, a Department of Biological Sciences, University of Alberta, Edmonton, Alberta T6G 2E9, Canada b Department of Botany, University of Wisconsin, Madison, WI 53706, USA article info abstract Article history: Brassicales is a diverse order perhaps most famous because it houses Brassicaceae and, its premier mem- Received 22 July 2015 ber, Arabidopsis thaliana. This widely distributed and species-rich lineage has been overlooked as a Revised 24 February 2016 promising system to investigate patterns of disjunct distributions and diversification rates. We analyzed Accepted 25 February 2016 plastid and mitochondrial sequence data from five gene regions (>8000 bp) across 151 taxa to: (1) Available online 15 March 2016 produce a chronogram for major lineages in Brassicales, including Brassicaceae and Arabidopsis, based on greater taxon sampling across the order and previously overlooked fossil evidence, (2) examine Keywords: biogeographical ancestral range estimations and disjunct distributions in BioGeoBEARS, and (3) determine Arabidopsis thaliana where shifts in species diversification occur using BAMM. The evolution and radiation of the Brassicales BAMM BEAST began 103 Mya and was linked to a series of inter-continental vicariant, long-distance dispersal, and land BioGeoBEARS bridge migration events. North America appears to be a significant area for early stem lineages in the Brassicaceae order. Shifts to Australia then African are evident at nodes near the core Brassicales, which diverged Cleomaceae 68.5 Mya (HPD = 75.6–62.0). -
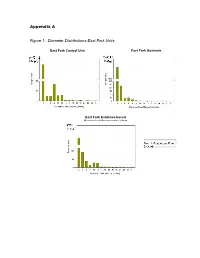
Appendix a Figure 1: Diameter Distributions-East Fork Units
Appendix A Figure 1: Diameter Distributions-East Fork Units East Fork Control Unit East Fork Goshawk Guidelines Unit East Fork Evidence-based Ecological Restoration Unit Figure 2: Diameter Distributions-Redondo Units Redondo Control Unit Redondo Goshawk Guidelines Unit Redondo Evidence-based Ecological Restoration Unit Figure 3: FlamMap Simulated Crown Fire Behavior: East Fork Project Area 20 mph Southwest Wind 25 mph 30 mph 35 mph 40 mph Map Legend Crown Fire Activity N No crown fire Passive crown activity fire (torching) Active crown fire (spreading) Important Note: Expected Crown Fire Behavior by Percent The 97th percentile of low fuel of Project Area moistures for June from 34 No Crown Passive years of data from the Kaibab Fire Crown Fire Active National Forest, Arizona were used in all simulations. This Windspeed Activity (torching) Crown Fire 20 79.9 0.9 19.2 input represents potential 25 71.2 1.9 26.9 extremes of fire weather during the peak of the fire 30 64.9 2.9 32.3 season, not average fire 35 57.3 4.7 38.0 season conditions. 40 48.7 9.4 42.0 Figure 4: FlamMap Simulated Crown Fire Behavior: Redondo Project Area 20 mph Southwest Wind 25 mph 30 mph 35 mph 40 mph Map Legend Crown Fire Activity N Highway 4 Valles Caldera No crown fire Passive crown activity entrance road fire (torching) Active crown fire (spreading) Important Note: Expected Crown Fire Behavior by Percent The 97th percentile of low fuel of Project Area moistures for June from 34 Passive years of data from the Kaibab No Crown Crown Fire Active Crown National Forest, Arizona were Windspeed Fire Activity (torching) Fire used in all simulations. -

Expression Profiling and Local Adaptation of Boechera Holboellii
CORE Metadata, citation and similar papers at core.ac.uk Provided by DigitalCommons@CalPoly µBlackwellExpression Publishing Ltd profiling and local adaptation of Boechera holboellii populations for water use efficiency across a naturally occurring water stress gradient CHARLES A. KNIGHT HEIKO VOGEL JUERGEN KROYMANN ALICE SHUMATE HANNEKE WITSENBOER and THOMAS MITCHELL-OLDS Abstract We studied the physiological basis of local adaptation to drought in Boechera holboellii, a perennial relative of Arabidopsis thaliana, and used cDNA–AFLPs to identify candidate genes showing differential expression in these populations. We compared two populations of B. holboellii from contrasting water environments in a reciprocal transplant experiment, as well as in a laboratory dry-down experiment. We continuously measured the water con tent of soils using time domain reflectometery (TDR). We compared populations for their water use efficiency (WUE), root/shoot ratios (R:S) and leaf mass per unit area (LMA) in the field and in the laboratory, and identified candidate genes that (i) responded plastically to water stress and (ii) were differentially expressed between the two populations. Genotypes from the drier site had higher WUE, which was attributable to a large reduction in transpi rational water loss. The xeric-adapted population also had increased investment in root bio mass and greater leaf mass per unit area. Reciprocal transplants in the field had significantly greater survival in their native habitat. In total, 450 cDNA-AFLP fragments showed significant changes between drought and control treatments. Furthermore, some genes showed genotype (population)-specific patterns of up- or down-regulation in response to drought. Three hundred cDNA-AFLP bands were sequenced leading to the identification of cDNAs coding for proteins involved in signal transduction, transcriptional regulation, redox regulation, oxidative stress and pathways involved in stress adaptation. -

Index of Botanist Names Associated with the Flora of Putnam Park Frederick Warren King
Index of Botanist Names Associated with the Flora of Putnam Park Frederick Warren King Standard abbreviation form refers to how the botanist’s name may appear in the citation of a species. For a number of the botanists who appear below, they are the authorities or co- authorities for the names of many additional species. The focus in this list is on flowers that appear in Putnam Park. Andrews, Henry Cranke (c. 1759 – 1830). English botanist, botanical artist, and engraver. He is the authority for Scilla siberica, Siberian Squill. Standard abbreviation form: Andrews Aiton, William (1731–1793). He was a Scottish botanist, appointed director of Royal Botanic Gardens, Kew in 1759. He is the authority for Solidago nemoralis, Vaccinium angustifolium, Viola pubescens, and Viola sagittate. He is the former authority for Actaea rubra and Clintonia borealis. Standard abbreviation form: Aiton Aiton, William Townsend (1766 – 1849). English botanist, son of William Aiton. He is the authority for Barbarea vulgaris, Winter Cress. Standard abbreviation form: W.T. Aiton Al-Shehbaz, Ihsan Ali (b. 1939). Iraqi born American botanist, Senior Curator at the Missouri Botanical Garden. Co-authority for Arabidopsis lyrate, Lyre-leaved Rock Cress and Boechera grahamii, Spreading-pod Rock Cress, and authority for Boechera laevigata, Smooth Rock Cress. Standard abbreviation form: Al-Shehbaz Avé-Lallemant, Julius Léopold Eduard (1803 – 1867). German botanist, co-authority for Thalictrum dasycarpum, Tall Meadow Rue. The genus Lallemantia is named in his honor. Standard abbreviation form: Avé-Lall. Barnhart, John Hendley (1871 – 1949). Was an American botanist and non-practicing MD. He is the authority for Ratibida pinnata. -

Washington Flora Checklist a Checklist of the Vascular Plants of Washington State Hosted by the University of Washington Herbarium
Washington Flora Checklist A checklist of the Vascular Plants of Washington State Hosted by the University of Washington Herbarium The Washington Flora Checklist aims to be a complete list of the native and naturalized vascular plants of Washington State, with current classifications, nomenclature and synonymy. The checklist currently contains 3,929 terminal taxa (species, subspecies, and varieties). Taxa included in the checklist: * Native taxa whether extant, extirpated, or extinct. * Exotic taxa that are naturalized, escaped from cultivation, or persisting wild. * Waifs (e.g., ballast plants, escaped crop plants) and other scarcely collected exotics. * Interspecific hybrids that are frequent or self-maintaining. * Some unnamed taxa in the process of being described. Family classifications follow APG IV for angiosperms, PPG I (J. Syst. Evol. 54:563?603. 2016.) for pteridophytes, and Christenhusz et al. (Phytotaxa 19:55?70. 2011.) for gymnosperms, with a few exceptions. Nomenclature and synonymy at the rank of genus and below follows the 2nd Edition of the Flora of the Pacific Northwest except where superceded by new information. Accepted names are indicated with blue font; synonyms with black font. Native species and infraspecies are marked with boldface font. Please note: This is a working checklist, continuously updated. Use it at your discretion. Created from the Washington Flora Checklist Database on September 17th, 2018 at 9:47pm PST. Available online at http://biology.burke.washington.edu/waflora/checklist.php Comments and questions should be addressed to the checklist administrators: David Giblin ([email protected]) Peter Zika ([email protected]) Suggested citation: Weinmann, F., P.F. Zika, D.E. Giblin, B. -

A Vascular Flora Inventory
A Vascular Flora Inventory Ottawa Sands Ottawa County Parks, Michigan September 2020 Prepared by William Martinus & Associates Financial assistance for this project was provided, in part, by the Coastal Management Program, Water Resources Division, Michigan Department of Environment, Great Lakes, and Energy, under the National Coastal Zone Management program, through a grant from the National Oceanic and Atmospheric Administration, U.S. Department of Commerce. The statements, findings, conclusions, and recommendations in this report are those of the Ottawa County Parks & Recreation Commission and do not necessarily reflect the views of the Michigan Department of Environment, Great Lakes, and Energy or the National Oceanic and Atmospheric Administration. 1 Table of Contents I. Introduction and Purpose 3 II. Overview 3 III. Plant Communities 4 IV. Endangered, Threatened, and Special Concern Species 5 V. Species Lists 7 VI. References 21 2 I. Introduction and Purpose Ottawa Sands, Ottawa County Parks, consists of 345 acres including an 80-acre inland lake, natural forests, coastal dunes, intermittent wetlands, inundated shrub swamp, and riparian marsh, shrub, and swamp communities. The eleven natural plant communities occurring on the site are listed along with hundreds of associated plant and animal species. - Ottawa Sands is located near the mouth of the Grand River in sections 17, 18 and 20 of Spring Lake Township, Ottawa County, in Western Michigan. - Property includes 5,585 feet of Grand River frontage. - A Floristic Quality Assessment demonstrates that a diverse and extremely high-quality plant component exists at Ottawa Sands. Purpose - To gain an understanding of the vegetative plant communities and flora of western Ottawa County and central west Michigan area.