Interrelationships Between the Thyroid Gland and Adrenal Cortex During Fear, Cold and Restraint in the Sheep
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Expression Pattern of Delta-Like 1 Homolog in Developing Sympathetic Neurons and Chromaffin Cells
Published in "Gene Expression Patterns 30: 49–54, 2018" which should be cited to refer to this work. Expression pattern of delta-like 1 homolog in developing sympathetic neurons and chromaffin cells ∗ Tehani El Faitwria,b, Katrin Hubera,c, a Institute of Anatomy & Cell Biology, Albert-Ludwigs-University Freiburg, Albert-Str. 17, 79104, Freiburg, Germany b Department of Histology and Anatomy, Faculty of Medicine, Benghazi University, Benghazi, Libya c Department of Medicine, University of Fribourg, Route Albert-Gockel 1, 1700, Fribourg, Switzerland ABSTRACT Keywords: Delta-like 1 homolog (DLK1) is a member of the epidermal growth factor (EGF)-like family and an atypical notch Sympathetic neurons ligand that is widely expressed during early mammalian development with putative functions in the regulation Chromaffin cells of cell differentiation and proliferation. During later stages of development, DLK1 is downregulated and becomes DLK1 increasingly restricted to specific cell types, including several types of endocrine cells. DLK1 has been linked to Adrenal gland various tumors and associated with tumor stem cell features. Sympathoadrenal precursors are neural crest de- Organ of Zuckerkandl rived cells that give rise to either sympathetic neurons of the autonomic nervous system or the endocrine Development ffi Neural crest chroma n cells located in the adrenal medulla or extraadrenal positions. As these cells are the putative cellular Phox2B origin of neuroblastoma, one of the most common malignant tumors in early childhood, their molecular char- acterization is of high clinical importance. In this study we have examined the precise spatiotemporal expression of DLK1 in developing sympathoadrenal cells. We show that DLK1 mRNA is highly expressed in early sympa- thetic neuron progenitors and that its expression depends on the presence of Phox2B. -

I. Introduction B. Adrenal Cortex
I. Introduction Adrenal Glands • suprarenal – they sit on top of the kidneys • each is composed of 2 distinct regions: A. Adrenal Medulla - the inner region - comprises 20% of the gland - secretes epinephrine and norepinephrine - derived from ectoderm B. Adrenal Cortex 1) Zona Glomerulosa (outermost region) - produces mineralocorticoids (aldosterone) • the outer region 2) Zona Fasiculata (middle region) - produces glucocorticoids (cortisol) as well as • comprises 80% of the gland estrogens and androgens • secretes corticosteroids 3) Zona Reticularis (innermost region) • derived from mesoderm - same function as zona fasiculata DHEA – dehydroepiandrosterone • an adrenal androgen in females • responsible for growth of pubic and axillary hair C. Pathologies Associated with Adrenal II. Mineralocorticoids (Aldosterone) Androgen Hypersecretion A. Functions 1.Adrenogenital Syndrome - promotes reabsorption of Na+ and - hypersecretion of androgens or estrogens secretion of K+ from the distal portion of the a) in the adult female: nephron..primary regulator of salt balance and extracellular volume - masculinization (i.e. hirsutism) -Similar (but less important) effect on salt b) in the female embryo: transport in colon, salivary glands, and - female pseudohermaphroditism sweat glands. c) in the adult male: - no effect d) in young boys: - precocious pseudopuberty 1 II. Mineralocorticoids (Aldosterone) C. Pathologies B. Regulation of Secretion 1. Hypersecretion 1. Renin Angiotensin a. primary hyperaldosteronism - Angiotensin II stimulates aldost. secretion - Conn’s syndrome 2. Potassium - usually due to a tumor on the gland + - high levels of K induce aldost. secretion - too much secretion of gland itself 3. ACTH –no direct role b. secondary hyperaldosteronism - default in renin angiotensin system - most common in atherosclerosis of renal arteries C. Pathologies III. Glucocorticoids (Cortisol) 1. -
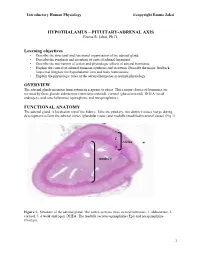
HYPOTHALAMUS – PITUITARY-ADRENAL AXIS Learning Objectives OVERVIEW FUNCTIONAL ANATOMY
Introductory Human Physiology ©copyright Emma Jakoi HYPOTHALAMUS – PITUITARY-ADRENAL AXIS Emma R. Jakoi, Ph.D. Learning objectives • Describe the structural and functional organization of the adrenal gland. • Describe the synthesis and secretion of cortical adrenal hormones. • Describe the mechanism of action and physiologic effects of adrenal hormones. • Explain the control of adrenal hormone synthesis and secretion. Describe the major feedback loops that integrate the hypothalamic axis and body homeostasis. • Explain the physiologic roles of the adrenal hormones in normal physiology. OVERVIEW The adrenal glands maintain homeostasis in response to stress. Three major classes of hormones are secreted by these glands: aldosterone (mineralocorticoid), cortisol (glucocorticoid), DHEA (weak androgen), and catecholamines (epinephrine and norepinephrine). FUNCTIONAL ANATOMY The adrenal gland is located on top of the kidney. Like the pituitary, two distinct tissues merge during development to form the adrenal cortex (glandular tissue) and medulla (modified neuronal tissue) (Fig 1). 1 2 cortex 3 medulla Figure 1. Structure of the adrenal gland. The cortex secretes three steroid hormones: 1. aldosterone, 2. cortisol, 3. a weak androgen, DHEA. The medulla secretes epinephrine (Epi) and norepinephrine (NorEpi). 1 Introductory Human Physiology ©copyright Emma Jakoi MINERALOCORTICOIDS The major mineralocorticoid in humans is aldosterone. Aldosterone is NOT under the hypothalamus- pituitary control and does not mediate a negative feedback to this axis. Aldosterone secretion is increased by the vasoconstrictor, angiotensin II, and by elevated plasma K+ concentration. Elevated plasma Na+ inhibits the secretion of aldosterone. Aldosterone, acts in the kidney to promote secretion of K+ into the urine from the blood and the reabsorption of Na+ from the urine into the blood. -

Beyond the Fixed Setpoint of the Hypothalamus–Pituitary–Thyroid Axis
E Fliers and others Hypothalamus–pituitary– 171:5 R197–R208 Review thyroid axis MECHANISMS IN ENDOCRINOLOGY Beyond the fixed setpoint of the hypothalamus–pituitary–thyroid axis Eric Fliers1, Andries Kalsbeek1,2 and Anita Boelen1 Correspondence should be addressed 1Department of Endocrinology and Metabolism, Academic Medical Center, University of Amsterdam, 1105 AZ to E Fliers Amsterdam, The Netherlands and 2Hypothalamic Integration Mechanisms, Netherlands Institute for Neuroscience, Email Amsterdam, The Netherlands e.fl[email protected] Abstract The hypothalamus–pituitary–thyroid (HPT) axis represents a classical example of an endocrine feedback loop. This review discusses dynamic changes in HPT axis setpoint regulation, identifying their molecular and cellular determinants, and speculates about their functional role. Hypothalamic thyrotropin-releasing hormone neurons were identified as key components of thyroid hormone (TH) setpoint regulation already in the 1980s, and this was followed by the demonstration of a pivotal role for the thyroid hormone receptor beta in negative feedback of TH on the hypothalamic and pituitary level. Gradually, the concept emerged of the HPT axis setpoint as a fixed entity, aiming at a particular TH serum concentration. However, TH serum concentrations appear to be variable and highly responsive to physiological and pathophysiological environmental factors, including the availability or absence of food, inflammation and clock time. During food deprivation and inflammation, TH serum concentrations decrease without a concomitant rise in serum TSH, reflecting a deviation from negative feedback regulation in the HPT axis. Surprisingly, TH action in peripheral organs in these conditions cannot be simply predicted by decreased serum TH concentrations. Instead, diverse environmental stimuli have differential effects on local TH metabolism, e.g. -

Study Guide Medical Terminology by Thea Liza Batan About the Author
Study Guide Medical Terminology By Thea Liza Batan About the Author Thea Liza Batan earned a Master of Science in Nursing Administration in 2007 from Xavier University in Cincinnati, Ohio. She has worked as a staff nurse, nurse instructor, and level department head. She currently works as a simulation coordinator and a free- lance writer specializing in nursing and healthcare. All terms mentioned in this text that are known to be trademarks or service marks have been appropriately capitalized. Use of a term in this text shouldn’t be regarded as affecting the validity of any trademark or service mark. Copyright © 2017 by Penn Foster, Inc. All rights reserved. No part of the material protected by this copyright may be reproduced or utilized in any form or by any means, electronic or mechanical, including photocopying, recording, or by any information storage and retrieval system, without permission in writing from the copyright owner. Requests for permission to make copies of any part of the work should be mailed to Copyright Permissions, Penn Foster, 925 Oak Street, Scranton, Pennsylvania 18515. Printed in the United States of America CONTENTS INSTRUCTIONS 1 READING ASSIGNMENTS 3 LESSON 1: THE FUNDAMENTALS OF MEDICAL TERMINOLOGY 5 LESSON 2: DIAGNOSIS, INTERVENTION, AND HUMAN BODY TERMS 28 LESSON 3: MUSCULOSKELETAL, CIRCULATORY, AND RESPIRATORY SYSTEM TERMS 44 LESSON 4: DIGESTIVE, URINARY, AND REPRODUCTIVE SYSTEM TERMS 69 LESSON 5: INTEGUMENTARY, NERVOUS, AND ENDOCRINE S YSTEM TERMS 96 SELF-CHECK ANSWERS 134 © PENN FOSTER, INC. 2017 MEDICAL TERMINOLOGY PAGE III Contents INSTRUCTIONS INTRODUCTION Welcome to your course on medical terminology. You’re taking this course because you’re most likely interested in pursuing a health and science career, which entails proficiencyincommunicatingwithhealthcareprofessionalssuchasphysicians,nurses, or dentists. -

Dream Recall/Affect and the Hypothalamic–Pituitary–Adrenal Axis
Review Dream Recall/Affect and the Hypothalamic–Pituitary–Adrenal Axis Athanasios Tselebis 1, Emmanouil Zoumakis 2 and Ioannis Ilias 3,* 1 Department of Psychiatry, Sotiria Hospital, 115 27 Athens, Greece; [email protected] 2 First Department of Pediatrics, National and Kapodistrian University of Athens Medical School, Aghia Sophia Children’s Hospital, 115 27 Athens, Greece; [email protected] 3 Department of Endocrinology, Diabetes and Metabolism, Elena Venizelou Hospital, 115 21 Athens, Greece * Correspondence: [email protected]; Tel.: +30-213-205-1389 Abstract: In this concise review, we present an overview of research on dream recall/affect and of the hypothalamic–pituitary–adrenal (HPA) axis, discussing caveats regarding the action of hormones of the HPA axis (mainly cortisol and its free form, cortisol-binding globulin and glucocorticoid receptors). We present results of studies regarding dream recall/affect and the HPA axis under physiological (such as waking) or pathological conditions (such as in Cushing’s syndrome or stressful situations). Finally, we try to integrate the effect of the current COVID-19 situation with dream recall/affect vis-à-vis the HPA axis. Keywords: dreams; cortisol; stress; memory; sleep; HPA axis 1. Introduction—Dream Recall Almost all humans dream (indeed, there may be 0.5% of people who do not do so) [1]. Dreams are a series of images, thoughts and senses and are considered a specific type Citation: Tselebis, A.; Zoumakis, E.; of experience that occurs in our brain during sleep. During the dream, there is limited Ilias, I. Dream Recall/Affect and the control of the dream content, visual images and memory activation. -

Pig Gonads, Adrenal Glands and Brain C
Immunoreactive cytochrome P-45017\g=a\in rat and guinea- pig gonads, adrenal glands and brain C. Le Goascogne1, N. Sanan\l=e'\s1, M. Gou\l=e'\zou1, S. Takemori2, S. Kominami2, E. E. Baulieu1 and P. Robel1 1INSERM U33, Communications Hormonales, and Faculté de Médecine, Université Paris-sud, Lab Hormones F-94275 Bicêtre Cedex France 2 Faculty of Integrated Arts and Sciences, Hiroshima University, Hiroshima 730, Japan Summary. The cytochrome P-45017\g=a\-hydroxylase, 17\ar=r\20lyase (P-45017\g=a\) is the key enzyme responsible for the biosynthesis of androgens in steroidogenic organs. Its cellular localization has been examined with an immunohistochemical technique. In immature rat ovary, P-45017\g=a\was first detected in sparse interstitial cells on postnatal Day 8. The number of immunoreactive interstitial cells increased thereafter and the intensity of P-45017\g=a\staining in these cells was highest at 3 weeks of age. The intensity of staining then started to decline and was very faint at Day 35. From 6 weeks on, the distribution of immunoreactive P-45017\g=a\was of the adult type: it was detected exclusively in the thecal cells of the large antral, preovulatory, follicles. P-45017\g=a\was not detectable during pregnancy except on the day of parturition, when thecal cells were transiently immunoreactive. The staining had vanished 24 h after delivery. Human chorionic gonadotrophin (hCG), injected into immature females on Days 24 to 26, induced P-45017\g=a\prematurely in thecal cells. When injected on Days 12 to 14 of pregnancy, hCG also induced P-45017\g=a\in the thecal cells surrounding the largest follicles, whereas the interstitial and luteal cells were not immunostained. -

Adrenal Gland Hormones
CHAPTER 8 Adrenal Gland Hormones Devra K. Dang, PharmD, BCPS, CDE, FNAP | Trinh Pham, PharmD, BCOP | Jennifer J. Lee, PharmD, BCPS, CDE LEARNING OBJECTIVES KEY TERMS AND DEFINITIONS After completing this chapter, you should be able to ACTH (adrenocorticotropic hormone) — a hormone produced 1. Identify the hormones produced by the adrenal glands by the pituitary gland that stimulates 2. Describe the functions of mineralocorticoids and glucocorticoids in the body the adrenal cortex to produce glucocorticoids, mineralocorticoids, 3. Recognize the signs and symptoms of adrenal insuffi ciency and androgens. PART 4. Describe the pharmacological treatment of patients with acute and chronic adrenal Addison ’ s disease — a disorder insuffi ciency in which the adrenal glands do not produce enough steroid hormones. 3 5. Recognize the signs and symptoms of Cushing ’ s syndrome and the result of too Adenoma — a benign much cortisol (noncancerous) tumor of glandular 6. Describe the pharmacologic and nonpharmacologic management of patients with origin. Cushing ’ s syndrome Adrenal insuffi ciency — a term 7. List management strategies for administration of glucocorticoid and mineralocorti- referring to a defi ciency in the levels of adrenal hormones. coid therapy to avoid development of adrenal disorders Aldosterone — the hormone produced by the adrenal glands that regulates the balance of sodium, he adrenal glands are an integral part of the endocrine system, secreting water, and potassium concentrations in the body. T hormones that act throughout the body to regulate functions and promote Corticotropin-releasing homeostasis. In addition to the neurotransmitters epinephrine and norepineph- hormone (CRH) — a hormone rine, the corticosteroids secreted by the adrenal glands are vital to a wide released by the hypothalamus that variety of physiological processes. -

Thyroid and Polycystic Ovary Syndrome
S Gabersˇcˇek and others Thyroid and PCOS 172:1 R9–R21 Review MECHANISMS IN ENDOCRINOLOGY Thyroid and polycystic ovary syndrome Simona Gabersˇcˇek1,2, Katja Zaletel1, Verena Schwetz3, Thomas Pieber3, Barbara Obermayer-Pietsch3 and Elisabeth Lerchbaum3 Correspondence should be addressed to 1Department of Nuclear Medicine, University Medical Centre Ljubljana, Zalosˇka 7, 1525 Ljubljana, Slovenia, B Obermayer-Pietsch 2Faculty of Medicine, University of Ljubljana, Vrazov trg 2, 1104 Ljubljana, Slovenia and 3Division of Endocrinology Email and Metabolism, Department of Internal Medicine, Medical University of Graz, Auenbruggerplatz 15, barbara.obermayer@ 8036 Graz, Austria medunigraz.at Abstract Thyroid disorders, especially Hashimoto’s thyroiditis (HT), and polycystic ovary syndrome (PCOS) are closely associated, based on a number of studies showing a significantly higher prevalence of HT in women with PCOS than in controls. However, the mechanisms of this association are not as clear. Certainly, genetic susceptibility contributes an important part to the development of HT and PCOS. However, a common genetic background has not yet been established. Polymorphisms of the PCOS-related gene for fibrillin 3 (FBN3) could be involved in the pathogenesis of HT and PCOS. Fibrillins influence the activity of transforming growth factor beta (TGFb). Multifunctional TGFb is also a key regulator of immune tolerance by stimulating regulatory T cells (Tregs), which are known to inhibit excessive immune response. With lower TGFb and Treg levels, the autoimmune processes, well known in HT and assumed in PCOS, might develop. In fact, lower levels of TGFb1 were found in HT as well as in PCOS women carrying allele 8 of D19S884 in the FBN3 gene. -

Thyroid Disease
Thyroid Disease Thyroid disease occurs when the thyroid (a small, butterfly-shaped gland in the front of your neck) does not produce the right amount of thyroid hormone. These hormones control how your body uses energy. If you are feeling fatigued, notice skin or hair changes, or have hoarseness or pain, your doctor may conduct a physical exam and order blood tests. If these tests indicate a problem, your doctor may order thyroid scan and uptake, thyroid biopsy, or imaging tests to help diagnose and evaluate a thyroid condition. Treatment will depend on the specific nature of your thyroid condition and its underlying cause. What is thyroid disease? The thyroid is a small, butterfly-shaped gland in the front of your neck that wraps around your windpipe (trachea). The two halves of the thyroid gland are connected in the middle by a thin layer of tissue known as the isthmus. The thyroid gland uses iodine (mostly absorbed from food) to produce hormones that control how your body uses energy. Nearly every organ in the body is affected by the function of the thyroid gland. The pituitary gland and hypothalamus, an area at the base of the brain, control the rate at which the thyroid produces and releases these hormones. The main function of the thyroid gland is to release a hormone called thyroxine or T4, which is converted into a hormone called T3. Both of these hormones circulate in the bloodstream and help regulate your metabolism. The amount of T4 produced by the thyroid gland is determined by a hormone produced by the pituitary gland called TSH or thyroid-stimulating hormone. -

Adrenal and Paraganglia Tumors Adrenal Cortical Tumors IHC: (+) SF1, Inhibin, Melan-A, Calretinin, Synaptophysin, (-) Chromogranin, Cytokeratin, S100
Last updated: 11/11/2020 Prepared by Kurt Schaberg Adrenal and Paraganglia Tumors Adrenal Cortical Tumors IHC: (+) SF1, Inhibin, Melan-A, Calretinin, Synaptophysin, (-) Chromogranin, Cytokeratin, S100. Often variable!! Adrenal Cortical Adenoma Benign. Very common. Often incidentally identified. Usually unilateral solitary masses with atrophic background adrenal gland. Tumor cells can be lipid-rich (clearer) or lipid-poor (pinker) arranged in nests and cords separated by abundant vasculature. Occasional lipofuscin pigment. Nuclei generally small and round (occasional extreme “endocrine atypia” is common). Low/no mitotic activity. Intact reticulin framework. On a spectrum with and may hard to differentiate from hyperplastic nodules, which is more often multinodular (background hyperplasia) and bilateral. Can be non-functional (85%) or functional (15%). Associated with MEN1, FAP, Carney Complex, among Aldosterone-producing→ “Conn syndrome”→ others… hypertension and hypokalemia If aldosterone-secreting adenoma is treated with Cortisol-producing→ (ACTH-independent) spironolactone→ “spironolactone bodies” (below) “Cushing Syndrome” → central obesity, moon face, hirsutism, poor healing, striae Sex-hormone-producing→ Rare (more common in carcinomas). Symptoms depend on hormone/sex (virilization or feminization) Adrenal Cortical Carcinoma Malignant. Most common in older adults. Can present with an incidental unilateral mass or with an endocrinopathy (see above). Solid, broad trabeculae, or large nested growth (more diffuse, and larger groups than in adenomas) Thick fibrous capsule with occasional fibrous bands. Frequent tumor necrosis. Frequent vascular or capsular invasion. Increased mitotic activity. Variants: Oncocytic, Myxoid, Sarcomatoid Mostly sporadic, but can be associated with Lynch Syndrome and Li-Fraumeni Syndrome Distinguishing between an Adrenal Cortical Adenoma vs Carcinoma Weiss Criteria: Weiss Criteria (≥3 = Malignant) Most widely used system, but doesn’t work as well High nuclear grade (based of Fuhrman criteria) in borderline cases or variants. -

Primary Hyperparathyroidism
Primary Hyperparathyroidism The parathyroid glands are 4 glands that reside within the thyroid glands: there are two parathyroid glands attached to each thyroid gland. The thyroid/parathyroid glands lie on either side of the trachea (wind pipe) about midway down the neck. The job of the parathyroid glands is to regulate calcium in the body. They secrete a hormone called parathyroid hormone (PTH), and this hormone draws calcium out of the bone where it is stored, as well as acting on the kidney and intestine to increase blood calcium. This is a normal process in response to fluctuations in blood calcium levels. The calcium in the blood is regulated by PTH (increases blood calcium) and calcitonin (a hormone secreted by the thyroid gland to decrease calcium) in order to keep it within a normal range. When the calcium gets too high or too low, symptoms arise. The most common symptoms of high calcium are increased thirst and urination. Other signs include: lethargy, weakness, diarrhea, inappetance, weight loss, tremors or stiffness. In about 50% of dogs with high calcium, urinary stones +/- urinary tract infection is present. Tumors (enlargement) of the parathyroid gland are typically not malignant, but they do over-secrete PTH. In 90% of cases only one of the four glands is enlarged. By one gland enlarging and over-secreting PTH, the three other glands typically shrink and temporarily stop functioning. Parathyroid tumors are suspected based on consistent blood work that shows elevated or normal PTH in the face of elevated calcium (the normal feedback mechanism would mean that when calcium is high, PTH is not needed and therefore is low).