Chapter 14: Ethers and Epoxides; Thiols and Sulfides 14.1 Introduction to Ethers – an Ether Group Is an Oxygen Atom That Is Bonded to Two Carbons
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

SAFETY DATA SHEET Isopropyl Alcohol
SAFETY DATA SHEET Isopropyl Alcohol Section 1. Identification GHS product identifier : Isopropyl Alcohol Chemical name : Isopropyl alcohol Other means of : isopropanol; 2-Propanol identification Product type : Liquid. Product use : Synthetic/Analytical chemistry. Synonym : isopropanol; 2-Propanol SDS # : 001105 Supplier's details : Airgas USA, LLC and its affiliates 259 North Radnor-Chester Road Suite 100 Radnor, PA 19087-5283 1-610-687-5253 24-hour telephone : 1-866-734-3438 Section 2. Hazards identification OSHA/HCS status : This material is considered hazardous by the OSHA Hazard Communication Standard (29 CFR 1910.1200). Classification of the : FLAMMABLE LIQUIDS - Category 2 substance or mixture EYE IRRITATION - Category 2A SPECIFIC TARGET ORGAN TOXICITY (SINGLE EXPOSURE) (Narcotic effects) - Category 3 GHS label elements Hazard pictograms : Signal word : Danger Hazard statements : May form explosive mixtures with air. Highly flammable liquid and vapor. Causes serious eye irritation. May cause drowsiness or dizziness. Precautionary statements General : Read label before use. Keep out of reach of children. If medical advice is needed, have product container or label at hand. Prevention : Wear protective gloves. Wear eye or face protection. Keep away from heat, hot surfaces, sparks, open flames and other ignition sources. No smoking. Use explosion- proof electrical, ventilating, lighting and all material-handling equipment. Use only non- sparking tools. Take precautionary measures against static discharge. Keep container tightly closed. Use only outdoors or in a well-ventilated area. Avoid breathing vapor. Wash hands thoroughly after handling. Response : IF INHALED: Remove person to fresh air and keep comfortable for breathing. Call a POISON CENTER or physician if you feel unwell. -

Physicochemical Properties of Organic Medicinal Agents
Principles of Drug Action 1, Spring 2005, Esters ESTERS AND RELATED CARBOXYLIC ACID DERIVATIVES Jack DeRuiter I. Structure and Preparation Esters are derivatives of carboxylic acids that arise via replacement of the hydroxyl (OH) portion of the acid COOH function with an "ether" moiety (-OR): O O H C C O C O Acid Ester Note that replacement of the acid OH group with an "ether" moiety removes the acidic function from the parent structure (acid) resulting in the formation of non-acidic (neutral, but somewhat polar) compounds (esters). Esters can be sub-classified based on their general structure as aliphatic, aromatic or cyclic (called "lactones") as illustrated by the examples below: O O CH2CH3 CH2CH3 O CH3 O O O Aliphatic Ester Aromatic Ester Cyclic Ester (Lactone) A variety of methods have been developed for the preparation of esters. Most of these methods involve reaction of an alcohol with an "activated carboxylic acid" compound (i.e. acid chloride): O O H C C X OC C O X- Ester "Activated" acid (X=Cl) Alcohol (Electrophile) (Nucleophile) The ester functionality does not introduce a center of asymmetry and thus optical and geometric isomerism does not result from the presence of this functional group. The ester functionality (the carbonyl and ether oxygen) is composed of an sp2 hybridized carbon so it cannot be chiral, and since there is free rotation about the ether bond geometric isomerism also is not possible at the sp2 center. 1 Principles of Drug Action 1, Spring 2005, Esters II. Solubility of Esters Esters contain carbonyl (C=O) and ether (O-C) dipoles arising from covalent bonding between electronegative oxygen atoms and electronically neutral carbon atoms. -

Halogenated Ether, Alcohol, and Alkane Anesthetics Activate TASK-3 Tandem Pore Potassium Channels Likely Through a Common Mechanism S
Supplemental material to this article can be found at: http://molpharm.aspetjournals.org/content/suppl/2017/03/21/mol.117.108290.DC1 1521-0111/91/6/620–629$25.00 https://doi.org/10.1124/mol.117.108290 MOLECULAR PHARMACOLOGY Mol Pharmacol 91:620–629, June 2017 Copyright ª 2017 by The American Society for Pharmacology and Experimental Therapeutics Halogenated Ether, Alcohol, and Alkane Anesthetics Activate TASK-3 Tandem Pore Potassium Channels Likely through a Common Mechanism s Anita Luethy, James D. Boghosian, Rithu Srikantha, and Joseph F. Cotten Department of Anesthesia, Critical Care, and Pain Medicine, Massachusetts General Hospital, Boston, Massachusetts (A.L., J.D.B., and J.F.C.); Department of Anesthesia, Kantonsspital Aarau, Aarau, Switzerland (A.L.); Carver College of Medicine, University of Iowa, Iowa City, Iowa (R.S.) Received January 7, 2017; accepted March 20, 2017 Downloaded from ABSTRACT The TWIK-related acid-sensitive potassium channel 3 (TASK-3; hydrate (165% [161–176]) . 2,2-dichloro- . 2-chloro 2,2,2- KCNK9) tandem pore potassium channel function is activated by trifluoroethanol . ethanol. Similarly, carbon tetrabromide (296% halogenated anesthetics through binding at a putative anesthetic- [245–346]), carbon tetrachloride (180% [163–196]), and 1,1,1,3,3,3- binding cavity. To understand the pharmacologic requirements for hexafluoropropanol (200% [194–206]) activate TASK-3, whereas molpharm.aspetjournals.org TASK-3 activation, we studied the concentration–response of the larger carbon tetraiodide and a-chloralose inhibit. Clinical TASK-3 to several anesthetics (isoflurane, desflurane, sevoflurane, agents activate TASK-3 with the following rank order efficacy: halothane, a-chloralose, 2,2,2-trichloroethanol [TCE], and chloral halothane (207% [202–212]) . -

BENZENE AS a LARVICIDE for SCREW WORMS1 the Larval Stage
BENZENE AS A LARVICIDE FOR SCREW WORMS1 By D. C. PARMAN Assistant Entomologist, Investigations of Insects Affecting the Health of Animals, Bureau of Entomology, United States Department of Agriculture INTRODUCTION The larval stage of CocMiomyia macellaria Fab., generally known amonff stock raisers in the Southwest as the screw worm, causes con- siderable loss to the livestock industry, estimated as high as $5,000,000 in some years. It has been apparent that the larvicides used to kill the worms are either toxic to the animal or at least in most cases detrimental to the healing of the wounds. This toxicity was at first attributed to the screw worm, but as many cases were ob- served where the animal was practically consumed by the larvae and still lived until the loss of olood or injury to some vital organ brought de^th, it was surmised that the treatments with larvicides were the cause of many deaths. During the summer of 1916 syste- matic work was begun to find a more efficient larvicide than the phenols and chloroform which were generally used. At first an attempt was made to add something to these larvicides to counteract the toxic properties. As this was not successful it was deemed best to look for a chemical that might be used with more satis- factory results. Several chemical groups were studied for possible larvicides. EXPERIMENTAL PROCEDURE All available chemicals with possible larvicidal value were selected for laboratory tests to determine whether they would kill the larvae of the screw-worm fly. The first tests were made by pouring the chemical on a number of larvae in a tube, or dusting on just enough to cover the larvae. -

Chemistry and Physics of Lipids Effects of Ether Vs. Ester Linkage On
Chemistry and Physics of Lipids 160 (2009) 33–44 Contents lists available at ScienceDirect Chemistry and Physics of Lipids journal homepage: www.elsevier.com/locate/chemphyslip Effects of ether vs. ester linkage on lipid bilayer structure and water permeability S. Deren Guler a, D. Dipon Ghosh b, Jianjun Pan a, John C. Mathai c, Mark L. Zeidel c, John F. Nagle a,b, Stephanie Tristram-Nagle a,∗ a Department of Physics, Carnegie Mellon University, Pittsburgh, PA 15213, USA b Department of Biological Sciences, Carnegie Mellon University, Pittsburgh, PA 15213, USA c Department of Medicine, Beth Israel Deaconess Medical Center and Harvard Medical School, Cambridge, MA 02139, USA article info abstract Article history: The structure and water permeability of bilayers composed of the ether-linked lipid, dihexadecylphos- Received 29 January 2009 phatidylcholine (DHPC), were studied and compared with the ester-linked lipid, dipalmitoylphosphadit- Received in revised form 29 March 2009 dylcholine (DPPC). Wide angle X-ray scattering on oriented bilayers in the fluid phase indicate that the Accepted 26 April 2009 area per lipid A is slightly larger for DHPC than for DPPC. Low angle X-ray scattering yields A = 65.1 Å2 for Available online 3 May 2009 ◦ −13 DHPC at 48 C. LAXS data provide the bending modulus, KC = 4.2 × 10 erg, and the Hamaker parame- ter H =7.2× 10−14 erg for the van der Waals attractive interaction between neighboring bilayers. For the Keywords: low temperature phases with ordered hydrocarbon chains, we confirm the transition from a tilted L Ether lipid ◦ gel phase to an untilted, interdigitated LI phase as the sample hydrates at 20 C. -

N-BUTYL ALCOHOL
Right to Know Hazardous Substance Fact Sheet Common Name: n-BUTYL ALCOHOL Synonyms: Propyl Carbinol; n-Butanol CAS Number: 71-36-3 Chemical Name: 1-Butanol RTK Substance Number: 1330 Date: November 1998 Revision: January 2008 DOT Number: UN 1120 Description and Use EMERGENCY RESPONDERS >>>> SEE BACK PAGE n-Butyl Alcohol is a colorless liquid with a strong, sweet Hazard Summary alcohol odor. It is used as a solvent for fats, waxes, shellacs, Hazard Rating NJDOH NFPA resins, gums, and varnish, in making hydraulic fluids, and in HEALTH - 2 medications for animals. FLAMMABILITY - 3 REACTIVITY - 0 f ODOR THRESHOLD = 1 to 15 ppm FLAMMABLE f Odor thresholds vary greatly. Do not rely on odor alone to POISONOUS GASES ARE PRODUCED IN FIRE determine potentially hazardous exposures. CONTAINERS MAY EXPLODE IN FIRE Hazard Rating Key: 0=minimal; 1=slight; 2=moderate; 3=serious; Reasons for Citation 4=severe f n-Butyl Alcohol is on the Right to Know Hazardous f n-Butyl Alcohol can affect you when inhaled and by Substance List because it is cited by OSHA, ACGIH, DOT, passing through the skin. NIOSH, DEP, IRIS, NFPA and EPA. f Contact can irritate and burn the skin. f This chemical is on the Special Health Hazard Substance f n-Butyl Alcohol can irritate and burn the eyes with possible List. eye damage. f Inhaling n-Butyl Alcohol can irritate the nose, throat and lungs. f Exposure to n-Butyl Alcohol can cause headache, dizziness, nausea and vomiting. SEE GLOSSARY ON PAGE 5. f n-Butyl Alcohol can damage the liver, kidneys, hearing, and sense of balance. -
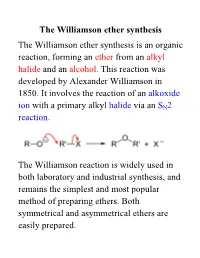
Williamson Ether Synthesis the Williamson Ether Synthesis Is an Organic Reaction, Forming an Ether from an Alkyl Halide and an Alcohol
The Williamson ether synthesis The Williamson ether synthesis is an organic reaction, forming an ether from an alkyl halide and an alcohol. This reaction was developed by Alexander Williamson in 1850. It involves the reaction of an alkoxide ion with a primary alkyl halide via an SN2 reaction. The Williamson reaction is widely used in both laboratory and industrial synthesis, and remains the simplest and most popular method of preparing ethers. Both symmetrical and asymmetrical ethers are easily prepared. The reaction for this week: an example of a Williamson ether synthesis acetaminophen ethyl iodide phenacetin starting material reagent product Phenacetin may be synthesized as an example of the Williamson ether synthesis The first synthesis of phenacetin was reported in 1878 by Harmon Morse. Procedure 1. Weigh an Extra-Strength Tylenol tablet. Pulverize the tablet with mortar and pestle. Weigh out 0.22 g and place it in a dry 15-ml round-bottom flask along with 0.28 g of finely pulverized K2CO3 (mortar and pestle) and 3.0 mL of butanone. Carefully add 0.28 mL of ethyl iodide with a syringe. 2. Add a stir bar; attach a microscale water-cooled condenser to the flask. Heat the mixture under reflux directly on a hot plate at medium setting for 1 hour. In the meantime, obtain the IR of acetaminophen. 3. Turn off the heat. Allow the mixture to cool down. Add 4 mL of water to the flask and transfer its contents to a 16 x 125 mm test tube with a screw cap. Rinse round-bottom flask 4 times with 1 mL of tert-butyl methyl ether (BME) and add the rinsings to the test tube. -

Reassessment of 3 Tolerance Exemptions for Ethylene Glycol
UNITED STATES ENVIRONMENTAL PROTECTION AGENCY - +,TE* sr4, WASHINGTON, D.C. 20460 Q c, OFFICE OF PREVENTION, PESTICIDES, AND TOXIC SUBSTANCES DATE: June 29,2006 ACTION MEMORANDUM SUBJECT: Reassessment of 3 Tolerance Exemptions for Ethylene Glycol, Diethylene Glycol, and the Combination of Diethylene Glycol Monomethyl Ether, Diethylene Glycol Monoethyl Ether, and Diethylene Glycol Monobutyl Ether FROM: Pauline Wagner, Chief F b.~!!<Lo 'v \ 3~~10 b Inert Ingredient Assessment Branch Registration Division (7505P) TO: Lois A. Rossi, Director Registration Division (7505P) 1. FQPA REASSESSMENT ACTION Action: Reassessment of three inert exemptions from the requirement of a tolerance. The reassessment decision is to maintain the inert tolerance exemptions "as-is." Table 1. Tolerance Exemptions Being Reassessed in this Document CM~fl,~aa,it Appeara in the CFR CAS iT01muw Registry Number @.I@,- Bxemption $in$@ Uses Name %SOa ,. Exprmsion. Antifreeze, deactivator for all pesticides 107-21-1 920 Ethylene glycol - - - used before crop emerges from soil and in 1,2-Ethanediol herbicides before or after crop emerges Deactivator, adjuvant for formulations used before crop emerges from soil and 11 1-46-6 920 Diethylene glycol --- deactivator for formulations used before Ethanol, 2,2'-oxybis- (9CI) crop emerges from soil, stabilizer Diethylene glycol 1 11-77-3 monomethyl ether Ethanol, 2-(2-methoxyethoxy)- 920 Diethylene glycol monoethyl - - - Deactivator for formulations used before 1 1 1-90-0 ether crop emerges from soil, stabilizer Ethanol, 2-(2-ethoxyethoxy)- Diethylene glycol monobutyl 112-34-5 ether Ethanol, 2-(2-butoxyethoxy)- a. Residues listed in 40 CFR 180.920 are exempted from the requirement of a tolerance when used in accordance with good agricultural practice as inert (or occasionally active) ingredients in pesticide formulations applied to growing crops only. -

How Is Alcohol Metabolized by the Body?
Overview: How Is Alcohol Metabolized by the Body? Samir Zakhari, Ph.D. Alcohol is eliminated from the body by various metabolic mechanisms. The primary enzymes involved are aldehyde dehydrogenase (ALDH), alcohol dehydrogenase (ADH), cytochrome P450 (CYP2E1), and catalase. Variations in the genes for these enzymes have been found to influence alcohol consumption, alcohol-related tissue damage, and alcohol dependence. The consequences of alcohol metabolism include oxygen deficits (i.e., hypoxia) in the liver; interaction between alcohol metabolism byproducts and other cell components, resulting in the formation of harmful compounds (i.e., adducts); formation of highly reactive oxygen-containing molecules (i.e., reactive oxygen species [ROS]) that can damage other cell components; changes in the ratio of NADH to NAD+ (i.e., the cell’s redox state); tissue damage; fetal damage; impairment of other metabolic processes; cancer; and medication interactions. Several issues related to alcohol metabolism require further research. KEY WORDS: Ethanol-to acetaldehyde metabolism; alcohol dehydrogenase (ADH); aldehyde dehydrogenase (ALDH); acetaldehyde; acetate; cytochrome P450 2E1 (CYP2E1); catalase; reactive oxygen species (ROS); blood alcohol concentration (BAC); liver; stomach; brain; fetal alcohol effects; genetics and heredity; ethnic group; hypoxia The alcohol elimination rate varies state of liver cells. Chronic alcohol con- he effects of alcohol (i.e., ethanol) widely (i.e., three-fold) among individ- sumption and alcohol metabolism are on various tissues depend on its uals and is influenced by factors such as strongly linked to several pathological concentration in the blood T chronic alcohol consumption, diet, age, consequences and tissue damage. (blood alcohol concentration [BAC]) smoking, and time of day (Bennion and Understanding the balance of alcohol’s over time. -

Reactions of Alkenes and Alkynes
05 Reactions of Alkenes and Alkynes Polyethylene is the most widely used plastic, making up items such as packing foam, plastic bottles, and plastic utensils (top: © Jon Larson/iStockphoto; middle: GNL Media/Digital Vision/Getty Images, Inc.; bottom: © Lakhesis/iStockphoto). Inset: A model of ethylene. KEY QUESTIONS 5.1 What Are the Characteristic Reactions of Alkenes? 5.8 How Can Alkynes Be Reduced to Alkenes and 5.2 What Is a Reaction Mechanism? Alkanes? 5.3 What Are the Mechanisms of Electrophilic Additions HOW TO to Alkenes? 5.1 How to Draw Mechanisms 5.4 What Are Carbocation Rearrangements? 5.5 What Is Hydroboration–Oxidation of an Alkene? CHEMICAL CONNECTIONS 5.6 How Can an Alkene Be Reduced to an Alkane? 5A Catalytic Cracking and the Importance of Alkenes 5.7 How Can an Acetylide Anion Be Used to Create a New Carbon–Carbon Bond? IN THIS CHAPTER, we begin our systematic study of organic reactions and their mecha- nisms. Reaction mechanisms are step-by-step descriptions of how reactions proceed and are one of the most important unifying concepts in organic chemistry. We use the reactions of alkenes as the vehicle to introduce this concept. 129 130 CHAPTER 5 Reactions of Alkenes and Alkynes 5.1 What Are the Characteristic Reactions of Alkenes? The most characteristic reaction of alkenes is addition to the carbon–carbon double bond in such a way that the pi bond is broken and, in its place, sigma bonds are formed to two new atoms or groups of atoms. Several examples of reactions at the carbon–carbon double bond are shown in Table 5.1, along with the descriptive name(s) associated with each. -

In This Handout, All of Our Functional Groups Are Presented As Condensed Line Formulas, 2D and 3D Formulas and with Nomenclature Prefixes and Suffixes (If Present)
In this handout, all of our functional groups are presented as condensed line formulas, 2D and 3D formulas and with nomenclature prefixes and suffixes (if present). Organic names are built on a foundation of alkanes, alkenes and alkynes. Those examples are presented first and you need to know those rules. The strategies can be found in Chapter 4 of our textbook (alkanes: pages 93-98, cycloalkanes 102-104, alkenes: pages 104-110, alkynes: pages 112-113 and combinations of all of them 113-115). After introducing examples of alkanes, alkenes, alkynes and combinations of them, the functional groups are presented in order of priority. A few nomenclature examples are provided for each of the functional groups. Examples of the various functional groups are presented on pages 115-135 in the textbook. Two overview pages are on pages 136-137. Some functional groups have a suffix name when they are the highest priority functional group and a prefix name when they are not the highest priority group, and these are added to the skeletal names with identifying numbers and stereochemistry terms (E and Z for alkenes, R and S for chiral centers and cis and trans for rings). Several low priority functional groups only have a prefix name. A few additional special patterns are shown on pages 98-102. The only way to learn this topic is practice (over and over). The best practice approach is to actually write out the names (on an extra piece of paper or on a white board, and then do it again). The same functional groups are used throughout the entire course. -

Phosphine-Catalyzed Additions of Nucleophiles and Electrophiles to Α
PHOSPHINE-CATALYZED ADDITIONS OF NUCLEOPHILES AND ELECTROPHILES TO α,β–UNSATURATED CARBONYL COMPOUNDS Reported by Michael Scott Bultman November 4, 2004 INTRODUCTION Organophosphorous compounds are becoming increasingly important in organic synthesis. Phosphines serve as precursors to phosphonium ylides in the Wittig reaction,1 and as nucleophilic triggers in the Mitsunobu2 and Staudinger3 reactions. In these processes, the phosphine is stoichiometrically consumed and converted into a phosphine oxide. Phosphines are also commonly used as ligands for transition metal-catalyzed reactions, to modulate reactivity and stereocontrol.4 On the other hand, the use of phosphines as nucleophilic catalysts for organic reactions has only gained attention in the last ten years. First reported by Rauhut and Currier in 1963,5 phosphine catalysis has since been reinvestigated after the phosphine ligands in some transition-metal-catalyzed reactions were found to be better catalysts than the metal/phosphine complexes alone!6 Phosphines are well suited for catalyzing the addition of both nucleophiles and electrophiles to electron deficient alkenes, alkynes, and allenes. Activation of these α,β-unsaturated carbonyl systems with the phosphine enables the formation of new bonds at the α-, β-, and γ-positions. This report will highlight these different modes of addition to α,β-unsaturated carbonyl systems under phosphine catalysis that allow for the formation of a wide array of products from a single class of substrates. GENERAL REACTIVITY OF PHOSPHINES Key characteristics required for successful nucleophilic catalysis lie in the balance of leaving group ability, nucleophilicity, and ease of ylid formation. Increasing leaving group ability can often be + correlated with decreasing basicity.