One • the Structure of Earth and Its Constituents
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Lecture 2A Structure and Composition of the Earth Plate Tectonics
Engineering and Environmental Geology (EART 3012) – 2014 Lecture 2A Structure and composition of the Earth Plate tectonics Dr Tom Raimondo See Marshak pg. 42–53; 78–100 Figures taken from Earth: Portrait of a Planet, WW Norton & Co. Course outline and introduction Sweet weekly homework Every week, there are regular tasks that must be completed. There are clear expectations about the amount of time you should spend studying this course. Contact time per Non-contact time per week week Lectures 2 hours 1–2 hours pre- reading and revision Practicals 2 hours 1 hour pre-reading Weekly quizzes - 30 mins to 1 hour eModules - 30 mins to 1 hour Textbook online - 30 mins to 1 hour resources Total 4 hours 4–5 hours Lecture 2A Why do I need to know all this stuff? . Knowing the structure and composition of the Earth forms the basis for all geological concepts . We need to have a understanding of how the Earth behaves as a whole, and what its properties are, before we can consider more specific Earth systems and cycles . Plate tectonics is the fundamental geological theory for how the Earth works and how we can predict its behaviour . We need to understand this theory to be able to understand and interpret a range of geological phenomena (e.g. earthquakes, volcanoes, tsunamis, landslides, etc.) Lecture 2A Lecture outline Part 1: Structure and composition of the Earth . Layers of the Earth: crust, mantle and core . Lithosphere and asthenosphere Part 2: Plate tectonics . What is a tectonic plate? . Types of plate boundaries . Other plate features . -

Preprint Arxiv:1806.10939, 2018
Solid Earth Discuss., https://doi.org/10.5194/se-2019-4 Manuscript under review for journal Solid Earth Discussion started: 15 January 2019 c Author(s) 2019. CC BY 4.0 License. Bayesian geological and geophysical data fusion for the construction and uncertainty quantification of 3D geological models Hugo K. H. Olierook1, Richard Scalzo2, David Kohn3, Rohitash Chandra2,4, Ehsan Farahbakhsh2,4, Gregory Houseman3, Chris Clark1, Steven M. Reddy1, R. Dietmar Müller4 5 1School of Earth and Planetary Sciences, Curtin University, GPO Box U1987, Perth, WA 6845, Australia 2Centre for Translational Data Science, University of Sydney, NSW 2006 Sydney, Australia 3Sydney Informatics Hub, University of Sydney, NSW 2006 Sydney, Australia 4EarthByte Group, School of Geosciences, University of Sydney, NSW 2006 Sydney, Australia Correspondence to: Hugo K. H. Olierook ([email protected]) 10 Abstract. Traditional approaches to develop 3D geological models employ a mix of quantitative and qualitative scientific techniques, which do not fully provide quantification of uncertainty in the constructed models and fail to optimally weight geological field observations against constraints from geophysical data. Here, we demonstrate a Bayesian methodology to fuse geological field observations with aeromagnetic and gravity data to build robust 3D models in a 13.5 × 13.5 km region of the Gascoyne Province, Western Australia. Our approach is validated by comparing model results to independently-constrained 15 geological maps and cross-sections produced by the Geological Survey of Western Australia. By fusing geological field data with magnetics and gravity surveys, we show that at 89% of the modelled region has >95% certainty. The boundaries between geological units are characterized by narrow regions with <95% certainty, which are typically 400–1000 m wide at the Earth’s surface and 500–2000 m wide at depth. -

Earth's Interior
11/7/2012 Please do the Audio Setup Wizard ! 11/7/12 Science Class Connect with Mrs. McFarland & Mr. Gluckin Earth’s Interior Ohio Academic Content Standards Today’s Class Agenda • Review Ground Rules for Earth and Space Sciences Classes The Universe • Earth’s Stats 9. Describe the interior structure of Earth and the Earth’s crust as • Composition of the Earth divided into tectonic plates riding on top of the slow moving currents of magma in the mantle. – Crust/Mantle/Core 11. Use models to analyze the size and shape of Earth, its surface and • Structures of the Earth its interior (e.g. globes, topographic maps and satellite images). – Lithosphere/Asthenosphere • Lithospheric Plates The Study Island lesson is due by 4pm on Thursday: SI 2e • State of matter and heat Student Centered Objectives – Convection Currents I will be able to describe the layers on the inside of the Earth. • Reminders I will be able use images to look at the Earth’s interior by composition. • Today’s Slides I will understand the different physical properties of the Earth’s layers. • Exit Ticket • Your Questions Ground rules Earth’s Stats • Please close all other apps & web pages. No Facebook, games, music, etc. • The Earth's mass is about 5.98 x 1024 kg. • No off‐topic chat • Be respectful of each other • Don’t share personal information • Earth is the densest planet in our Solar • I can see all chat … even “private chat” System (mass/volume). • Earth is made of several layers with different compositions and physical properties, like temperature, density, and the viscosity (“aka” ability to flow). -
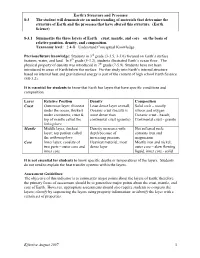
Earth's Structure and Processes 8-3 the Student Will Demonstrate An
Earth’s Structure and Processes 8-3 The student will demonstrate an understanding of materials that determine the structure of Earth and the processes that have altered this structure. (Earth Science) 8-3.1 Summarize the three layers of Earth – crust, mantle, and core – on the basis of relative position, density, and composition. Taxonomy level: 2.4-B Understand Conceptual Knowledge Previous/future knowledge: Students in 3rd grade (3-3.5, 3-3.6) focused on Earth’s surface features, water, and land. In 5th grade (5-3.2), students illustrated Earth’s ocean floor. The physical property of density was introduced in 7th grade (7-5.9). Students have not been introduced to areas of Earth below the surface. Further study into Earth’s internal structure based on internal heat and gravitational energy is part of the content of high school Earth Science (ES-3.2). It is essential for students to know that Earth has layers that have specific conditions and composition. Layer Relative Position Density Composition Crust Outermost layer; thinnest Least dense layer overall; Solid rock – mostly under the ocean, thickest Oceanic crust (basalt) is silicon and oxygen under continents; crust & more dense than Oceanic crust - basalt; top of mantle called the continental crust (granite) Continental crust - granite lithosphere Mantle Middle layer, thickest Density increases with Hot softened rock; layer; top portion called depth because of contains iron and the asthenosphere increasing pressure magnesium Core Inner layer; consists of Heaviest material; most Mostly iron and nickel; two parts – outer core and dense layer outer core – slow flowing inner core liquid, inner core - solid It is not essential for students to know specific depths or temperatures of the layers. -

Probing the Earth's Deep Interior Through Geochemistry
Probing the Earth’s Deep Interior Through Geochemistry White, W. M. (2015). Probing the Earth’s Deep Interior through Geochemistry. Geochemical Perspectives, 4(2), 95-251. European Association of Geochemistry Version of Record http://cdss.library.oregonstate.edu/sa-termsofuse Volume 4, number 2 | October 2015 Probing the Earth’s Deep Interior Through Geochemistry WILLIAM M. WHITE WILLIAM Each issue of Geochemical Perspectives pre- sents a single article with an in-depth view Editorial Board on the past, present and future of a field of geochemistry, seen through the eyes of highly Liane G. BenninG respected members of our community. The GFZ Postdam, Germany articles combine research and history of the University of Leeds, UK field’s development and the scientist’s opinions about future directions. We welcome personal glimpses into the author’s scientific life, how Janne BLicHerT-TofT ideas were generated and pitfalls along the way. ENS Lyon, France Perspectives articles are intended to appeal to the entire geochemical community, not only to experts. They are not reviews or monographs; they go beyond the current state of the art, im lliott providing opinions about future directions and T e University of Bristol, UK impact in the field. Copyright 2015 European Association of Geochemistry, EAG. All rights reserved. This journal and the individual contributions contained in it are protected under copy- right by the EAG. The following terms and conditions eric H. oeLkers apply to their use: no part of this publication may be University College London, UK reproduced, translated to another language, stored CNRS Toulouse, France in a retrieval system or transmitted in any form or by any means, electronic, graphic, mechanical, photo- copying, recording or otherwise, without prior written permission of the publisher. -

Geodynamics If the Entire Solid Earth Is Viewed As a Single Dynamic
http://www.paper.edu.cn Geodynamic Processes and Our Living Environment YANG Wencai, P. Robinson, FU Rongshan and WANG Ying Geological Publishing House: 2001 Geophysical System Yang Wencai, Institute of Geology, CAGS, China Key words: Geophysics, geodynamics, kinetics of the Earth, tectonics, energies of the Earth, driving forces, applied geophysics, sustainable development. Contents: I. About geophysical system II. Kinetics of the Earth III. About plate tectonics and plume tectonics IV. The contents in the topic V. Energies for the dynamic Earth VI. Driving Forces of Plate Tectonics VII. Geophysics and sustainable development of the society I. About geophysical system Rapid advance of sciences during the second half of the twentieth century has enabled man to make a successful star in the exploration of planetary space. The deep interior of the Earth, however, remains as inaccessible as ever. This is the realm of solid-Earth geophysics, which still mainly depends on observations made at or near the Earth's surface. Despite this limitation, a major revolution in knowledge of the Earth's interior has taken place over the last forty years. This has led to a new understanding of the processes which occur within the Earth that produce surface conditions outstandingly different from those of the other inner planets and the moon. How has this come about? It is mainly the results of the introduction of new experimental and computational techniques into geophysics. Geophysics as a major branch of the geosciences has been discussed in Topic 6.16.1. Theoretically and traditionally the geophysical system correlates to physical system, but specified st study the solid Earth, containing sub-branches such as gravitation and Earth-motion correlated with 转载 1 中国科技论文在线 http://www.paper.edu.cn mechanics, geothermics correlated with heat and thermics, seismology with acoustics and wave theory, geoelectricity and geomagnetism. -

Chapter 2 the Solid Materials of the Earth's Surface
CHAPTER 2 THE SOLID MATERIALS OF THE EARTH’S SURFACE 1. INTRODUCTION 1.1 To a great extent in this course, we will be dealing with processes that act on the solid materials at and near the Earth’s surface. This chapter might better be called “the ground beneath your feet”. This is the place to deal with the nature of the Earth’s surface materials, which in later sections of the chapter I will be calling regolith, sediment, and soil. 1.2 I purposely did not specify any previous knowledge of geology as a prerequisite for this course, so it is important, here in the first part of this chapter, for me to provide you with some background on Earth materials. 1.3 We will be dealing almost exclusively with the Earth’s continental surfaces. There are profound geological differences between the continents and the ocean basins, in terms of origin, age, history, and composition. Here I’ll present, very briefly, some basic things about geology. (For more depth on such matters you would need to take a course like “The Earth: What It Is, How It Works”, given in the Harvard Extension program in the fall semester of 2005– 2006 and likely to be offered again in the not-too-distant future.) 1.4 In a gross sense, the Earth is a layered body (Figure 2-1). To a first approximation, it consists of concentric shells: the core, the mantle, and the crust. Figure 2-1: Schematic cross section through the Earth. 73 The core: The core consists mostly of iron, alloyed with a small percentage of certain other chemical elements. -

Quantification of Water in Hydrous Ringwoodite
ORIGINAL RESEARCH ARTICLE published: 28 January 2015 EARTH SCIENCE doi: 10.3389/feart.2014.00038 Quantification of water in hydrous ringwoodite Sylvia-Monique Thomas 1*,StevenD.Jacobsen2, Craig R. Bina 2, Patrick Reichart 3, Marcus Moser 3, Erik H. Hauri 4, Monika Koch-Müller 5,JosephR.Smyth6 and Günther Dollinger 3 1 Department of Geoscience, University of Nevada Las Vegas, Las Vegas, NV, USA 2 Department of Earth and Planetary Sciences, Northwestern University, Evanston, IL, USA 3 Department für Luft- und Raumfahrttechnik LRT2, Universität der Bundeswehr München, Neubiberg, Germany 4 Department of Terrestrial Magnetism, Carnegie Institution of Washington, Washington, DC, USA 5 Helmholtz-Zentrum Potsdam, Deutsches GeoForschungsZentrum (GFZ), Potsdam, Germany 6 Department of Geological Sciences, University of Colorado Boulder, Boulder, CO, USA Edited by: Ringwoodite, γ-(Mg,Fe)2SiO4, in the lower 150 km of Earth’s mantle transition zone Mainak Mookherjee, Cornell (410–660 km depth) can incorporate up to 1.5–2wt% H2O as hydroxyl defects. We University, USA present a mineral-specific IR calibration for the absolute water content in hydrous Reviewed by: ringwoodite by combining results from Raman spectroscopy, secondary ion mass Geoffrey David Bromiley, University of Edinburgh, UK spectrometry (SIMS) and proton-proton (pp)-scattering on a suite of synthetic Mg- and Marc Blanchard, CNRS - Université Fe-bearing hydrous ringwoodites. H2O concentrations in the crystals studied here Pierre et Marie Curie, France range from 0.46 to 1.7wt% H2O (absolute methods), with the maximum H2Oin *Correspondence: the same sample giving 2.5 wt% by SIMS calibration. Anchoring our spectroscopic Sylvia-Monique Thomas, results to absolute H-atom concentrations from pp-scattering measurements, we report Department of Geoscience, University of Nevada Las Vegas, frequency-dependent integrated IR-absorption coefficients for water in ringwoodite −1 −2 4505 S. -

Clay Model Earth
Teacher Instructions Clay Model Earth Overview: During this lesson students will learn about Earth’s layers by making a clay model. As a result of this activity, students develop an understanding of the structure of Earth’s layers, particularly as it relates to thickness and solid or molten state. Students keep completed Earth models in the classroom for future reference during Units 2 and 3. Note: You may wish to build a sample model Earth in the classroom, then have students build models as a homework assignment. Objectives: The student will learn: • to identify Earth’s layers, including the inner core, outer core, mantle, and crust; • the relative size and thickness of each Earth layer; • that Earth’s inner core is solid due to tremendous pressure; and • that Earth’s outer core is molten, and exists in a “liquid” state. Materials: • Clay in the colors and amounts described in the activity procedure (see Note, following page) • Marbles (25 mm) for each student • Sharp knife • Ruler • Teacher Information Sheet: “The Thickness and State of Earth’s Layers” • Transparency: “Earth’s Layers” • Transparency: “Clay Model Earth” • Student Worksheet: “Clay Model Earth” Answers to Student Worksheet: Crust 1. refer to diagram 2. outer core Mantle 3. mantle Molten 4. crust outer core 5. This is a critical thinking question; answers will vary. Solid inner core Ola Ka Honua: Volcanoes Alive 43 ©2001, 2007 UAF Geophysical Institute Teacher Instructions Clay Model Earth Activity Procedure: 1. Give a 25 mm marble and the following amounts of each color clay to each student: • One 4 oz. -

High School - Earth and Space Science
High School - Earth and Space Science Grade Big Idea Essential Questions Concepts Competencies Vocabulary 2002 SAS Assessment Standards Standards Anchor Eligible Content The universe is What is the universe and The Milky Way Galaxy consists of Use models to describe the Clusters 3.4.10.D 3.3.10.B1 composed of a variety what is Earth’s place in more than two hundred billion sun’s place in space in relation Galaxy of different objects that it? stars, the sun being one of them, to the Milky Way Galaxy and the Model are organized into and is one of hundreds of billions distribution of galaxy clusters in Star 9-12 systems each of which of galaxies in the known universe. the universe. Universe develops according to accepted physical processes and laws. The universe is What is the universe and Models of the formation and Compare time periods in history, Geocentric 3.1.12.E 3.4.10.B composed of a variety what is Earth’s place in structure of the universe have the technology available at that Heliocentric 3.4.10.D3 of different objects that it? changed over time as technologies time and the resulting model of Model are organized into have become more advanced and the organization of our solar Planet 9-12 systems each of which the accuracy of our data has system. (e.g. – Early Greeks Theory develops according to increased. used purely observational data accepted physical resulting in a geocentric model). processes and laws. The universe is What is the universe and The Milky Way Galaxy consists of Use data about the expansion, Clusters 3.4.10.D 3.3.10.B1 composed of a variety what is Earth’s place in more than two hundred billion scale and age of the universe to Galaxy 3.3.12.B2 of different objects that it? stars, the sun being one of them, explain the Big Bang theory as a Light year are organized into and is one of hundreds of billions model for the origin of the Model 9-12 systems each of which of galaxies in the known universe. -

Framework and NGSS
POPULATIONS AND ECOSYSTEMS – Framework and NGSS INTRODUCTION TO Contents PERFORMANCE EXPECTATIONS Introduction to Performance “The NGSS are standards or goals, that reflect what a student Expectations .......................... 35 should know and be able to do; they do not dictate the manner or methods by which the standards are taught. Curriculum FOSS Conceptual and assessment must be developed in a way that builds students’ Framework ............................ 48 knowledge and ability toward the PEs [performance expectations]” Background for the (Next Generation Science Standards, 2013, page xiv). Conceptual Framework .......... 50 This chapter shows how the NGSS Performance Expectations are Connections to NGSS by bundled in the Populations and Ecosystems Course to provide Investigation .......................... 56 a coherent set of instructional materials for teaching and learning. This chapter also provides details about how this FOSS course Recommended FOSS Next fits into the matrix of the FOSS Program (page 49). Each FOSS Generation K–8 Scope and module K–5 and middle school course 6–8 has a functional role Sequence ............................... 74 in the FOSS conceptual frameworks that were developed based on a decade of research on science education and the influence of A Framework for K–12 Science Education (2012) and Next Generation Science Standards (NGSS, 2013). The FOSS curriculum provides a coherent vision of science teaching and learning in the three ways described by the The NGSS Performance Expectations NRC Framework. First, FOSS is designed around learning as bundled in this course include a developmental progression, providing experiences that allow students to continually build on their initial notions and develop Life Sciences more complex science and engineering knowledge. -

Planet Earth in Cross Section by Michael Osborn Fayetteville-Manlius HS
Planet Earth in Cross Section By Michael Osborn Fayetteville-Manlius HS Objectives Devise a model of the layers of the Earth to scale. Background Planet Earth is organized into layers of varying thickness. This solid, rocky planet becomes denser as one travels into its interior. Gravity has caused the planet to differentiate, meaning that denser material have been pulled towards Earth’s center. Relatively less dense material migrates to the surface. What follows is a brief description1 of each layer beginning at the center of the Earth and working out towards the atmosphere. Inner Core – The solid innermost sphere of the Earth, about 1271 kilometers in radius. Examination of meteorites has led geologists to infer that the inner core is composed of iron and nickel. Outer Core - A layer surrounding the inner core that is about 2270 kilometers thick and which has the properties of a liquid. Mantle – A solid, 2885-kilometer thick layer of ultra-mafic rock located below the crust. This is the thickest layer of the earth. Asthenosphere – A partially melted layer of ultra-mafic rock in the mantle situated below the lithosphere. Tectonic plates slide along this layer. Lithosphere – The solid outer portion of the Earth that is capable of movement. The lithosphere is a rock layer composed of the crust (felsic continental crust and mafic ocean crust) and the portion of the mafic upper mantle situated above the asthenosphere. Hydrosphere – Refers to the water portion at or near Earth’s surface. The hydrosphere is primarily composed of oceans, but also includes, lakes, streams and groundwater.