The Effect of Sewage Effluent Removal on the Water Quality of Lake Howell, Florida
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Special Publication SJ92-SP16 LAKE JESSUP RESTORATION
Special Publication SJ92-SP16 LAKE JESSUP RESTORATION DIAGNOSTIC EVALUATION HATER BUDGET AND NUTRIENT BUDGET Prepared for: ST. JOHNS RIVER WATER MANAGEMENT DISTRICT P.O. Box 1429 Palatka, Florida Prepared By: Douglas H. Keesecker HATER AND AIR RESEARCH, INC. Gainesville, Florida May 1992 File: 91-5057 TABLE OF CONTENTS (Page 1 of 2) Section Page EXECUTIVE SUMMARY i-1 1.0 INTRODUCTION 1-1 1.1 PURPOSE 1-1 1.2 DESCRIPTION OF THE STUDY AREA 1-2 2.0 DATA COMPILATION 2-1 2.1 HISTORICAL DATA 2-1 2.1.1 Previous Studies 2-1 2.1.2 Climatoloqic Data 2-3 2.1.3 Hydrologic Data 2-4 2.1.4 Land Use and Cover Data 2-6 2.1.5 On-site Sewage Disposal System (OSDS) Data 2-8 2.1.6 Wastewater Treatment Plant (WWTP1 Data 2-9 2.1.7 Surface Water Quality Data . 2-10 3.0 WATER BUDGET 3-1 3.1 METHODOLOGY 3-1 3.1.1 Springflow and Upward Leakage 3-2 3.1.2 Direct Precipitation 3-4 3.1.3 Land Surface Runoff 3-4 3.1.4 Shallow Groundwater Inflow 3-6 3.1.5 Septic Tank (OSDS1 Inflows 3-7 3.1.6 Wastewater Treatment Plant Effluent 3-8 3.1.7 Surface Evaporation 3-8 3.1.8 Surface Water Outflows 3-9 3.2 RESULTS - WATER BUDGET 3-10 3.3 DISCUSSION 3-16 3.4 ESTIMATE OF ERROR 3-18 3.4.1 Sprinoflow and Upward Leakage 3-18 3.4.2 Direct Precipitation 3-19 3.4.3 Land Surface Runoff 3-19 3.4.4 Shallow Groundwater Inflow 3-20 3.4.5 Septic Tank (OSDS1 Inflows 3-21 3.4.6 Wastewater Treatment Plant Effluent 3-22 3.4.7 Surface Evaporation 3-22 3.4.8 Surface Water Outflows 3-22 LAKE JESSUP[WP]TOC 052192 TABLE OF CONTENTS (Page 2 of 2) Section Page 4.0 NUTRIENT BUDGETS 4-1 4.1 METHODOLOGY 4-1 4.1.1 Direct Precipitation Loading 4-2 4.1.2 Land Surface Runoff Loading 4-3 4.1.3 Shallow Groundwater Inflow Loading 4-3 4.1.4 Septic Tank Loading 4-4 4.1.5 Wastewater Treatment Plant Loading 4-4 4.1.6 St. -

TMDL Report: Nutrient and Unionized Ammonia Tmdls for Lake Jesup, Wbids 2981 and 2981A
FINAL 4/14/06 FLORIDA DEPARTMENT OF ENVIRONMENTAL PROTECTION Division of Water Resource Management, Bureau of Watershed Management CENTRAL DISTRICT • MIDDLE ST. JOHNS BASIN TMDL Report Nutrient and Unionized Ammonia TMDLs for Lake Jesup, WBIDs 2981 and 2981A Xueqing Gao April 14, 2006 Executive Summary This report presents the Total Maximum Daily Load (TMDL) for nutrients and unionized ammonia for Lake Jesup (including Lake Jesup outlet), in the Middle St. Johns Basin. The lake was verified as impaired for nutrients and unionized ammonia due to elevated annual average Trophic State Index (TSI) values and exceedances of the unionized ammonia criterion (0.02 milligrams per liter [mg/L]), and was included on the Verified List of impaired waters for the Middle St. Johns Basin that was adopted by Secretarial Order on May 27, 2004. According to the 1999 Florida Watershed Restoration Act (FWRA), Chapter 99-223, Laws of Florida, once a waterbody is included on the Verified List, a TMDL must be developed. The purpose of the TMDL is to establish the allowable loadings of pollutants to Lake Jesup that would restore the waterbody so that it meets its applicable water quality criteria for nutrients and unionized ammonia. The nutrient TMDL for Lake Jesup was developed through four major steps: 1. Establish the TSI target 2. Model the existing nutrient load 3. Model the load that would achieve the TSI target (the TMDL) 4. Estimate the percent load reduction needed to meet the TMDL. To establish the TSI target, the Department first estimated the background TSI for the lake by comparing results from several methods, including a historic sedimentation rate method, literature published values, a TSI defined by the Florida Impaired Waters Rule, an Ecoregion approach, a hydrogeomorphologic method, and a model simulated background condition. -

OCTOBER 2018 • 1 OASOAS Is Is Orange Audubon Society the Voice of Conservation in Central Florida Vol
OCTOBER 2018 • 1 OASOAS is is Orange Audubon Society The Voice of Conservation in Central Florida Vol. 54 Issue 2 • October 2018 This Month’s Program: Fall Bird Walks: October 18, 2018 October 6, 13, 20, & 27, Coexisting with 2018, Mead Botanical Panthers Garden To celebrate fall migration, Orange by Dr. Jennifer Korn Although the Florida Panther is still Audubon Society (OAS) will host bird highly endangered, it is making a walks at Mead Botanical Garden on comeback, thanks to conserved land Saturdays, October 6, 13, 20 & 27, 2018. and conservation efforts. Most of the The October 20th walk will replace estimated 120 to 230 Florida Panthers the monthly field trip. The walks are live in South Florida. Orange Audubon free and open to the public with no Society’s October speaker, Dr. Jennifer registration needed. Each walk will be Korn, focuses on the ones in the rest of led by an experienced birding leader. the state. Walks start at 8:00 a.m. and will last 2 Previously only a few adventurous to 3 hours. The pace is slow with lots males crossed the Caloosahatchee of stopping to look at birds. River and traveled north in search of territory and mates, but in March 2017 a mother and kittens were documented on Babcock Ranch Preserve in Charlotte County — the first clear evidence of a female north of the Caloosahatchee River in over 40 years. Then Florida Fish and Wildlife Conservation Commission Dr. Jennifer Korn, tracking a panther at Archbold Biological Station. Photo: Dustin Angell (FWC) biologists documented a second female north of the river, Highlands Mead Garden, looking for a waterthrush. -

Assessment of Cyanotoxins in Florida's Lakes, Reservoirs And
Assessment of Cyanotoxins in Florida’s Lakes, Reservoirs and Rivers by Christopher D. Williams BCI Engineers and Scientists, Inc. Lakeland, FL. John W. Burns Andrew D. Chapman Leeanne Flewelling St. Johns River Water Management District Palatka, FL. Marek Pawlowicz Florida Department of Health/Bureau of Laboratories Jacksonville, FL. Wayne Carmichael Wright State University Dayton, OH. 2001 Executive Summary EXECUTIVE SUMMARY Harmful algal blooms (HABs) are population increases of algae above normal background levels and are defined by their negative impacts on the environment, the economy, and human health. Historically, many of Florida's largest and most utilized freshwater and estuarine systems have been plagued by occasional blooms of harmful algae. During the last decade, however, the frequency, duration, and concentration levels of these blooms in freshwater and brackish water have increased significantly, primarily due to changes in land utilization, changes in hydrology, increases in nutrient runoff, loss of aquatic vegetation, and a climate that is very conducive to algal growth and proliferation. In 1998, the Florida Harmful Algal Bloom Task Force was established to determine the extent to which HABs pose a problem for the state of Florida. Blue-green algae (cyanobacteria) were identified as top research priorities due to their potential to produce toxic chemicals and contaminate natural water systems. In June 1999, the St. Johns River Water Management District (SJRWMD) initiated a collaborative study in conjunction with the Florida Marine Research Institute, the Florida Department of Health, and Wright State University to determine the geographical distribution of various types of toxin-producing blue-green algae in Florida's surface waters and to positively identify any algal toxins present in these waters. -

5. CONSERVATION ELEMENT Data, Inventory, and Analysis
CITY OF ALTAMONTE SPRINGS CONSERVATION ELEMENT 5. CONSERVATION ELEMENT Data, Inventory, and Analysis INTRODUCTION The purpose of the Conservation Element is to promote the conservation, use, and protection of natural resources within the City of Altamonte Springs. The objective of the Conservation Element Data Inventory and Analysis (DIA) Report is to document the conditions for various natural resources found within the City and identify key community priorities for conservation strategies. The DIA Report also examines the current and projected water needs for the community over the 20-year planning horizon (2030) to ensure adequate water supply is available to support future demands. Together, this information serves as the foundation for goals, objectives, and policies prepared to guide future development within the City of Altamonte Springs. DATA AND ANALYSIS This section addresses the data inventory and analysis requirements of §9J-5.013(1), Florida Administrative Code (F.A.C.), supportive to the goals, objectives, policies and implementation programs for the Conservation Element. Natural resources may include water, wetlands, floodplains, groundwater, air, minerals, soil, wildlife, and vegetative resources. Surface Water Quality Surface water resources within the City of Altamonte Springs generally consist of the Little Wekiva River and several lakes. Little Wekiva River The Little Wekiva River flows north from Lake Lawne in Orange County through Lake Lotus and Trout Lake in Altamonte Springs. It continues to flow in a northerly direction at the City limits into one of the southern arms of the Wekiva Swamp and eventually on into the Wekiva River. Extensive urban development along the Little Wekiva River has resulted in a host of problems including increased rate of flow and velocities, minimal upstream stormwater storage and treatment due to development occurring before current stormwater regulations, erosion and flooding, and degradation of the river’s water quality from the movement and deposition of sediments. -

Florida Fish and Wildlife Conservation Commission Statewide Alligator Harvest Data Summary
FWC Home : Wildlife & Habitats : Managed Species : Alligator Management Program FLORIDA FISH AND WILDLIFE CONSERVATION COMMISSION STATEWIDE ALLIGATOR HARVEST DATA SUMMARY YEAR AVERAGE LENGTH TOTAL HARVEST FEET INCHES 2000 8 8 2,552 2001 8 8.2 2,268 2002 8 3.7 2,164 2003 8 4.6 2,830 2004 8 5.8 3,237 2005 8 4.9 3,436 2006 8 4.8 6,430 2007 8 6.7 5,942 2008 8 5.1 6,204 2009 8 0 7,844 2010 7 10.9 7,654 2011 8 1.2 8,103 Provisional data 2000 STATEWIDE ALLIGATOR HARVEST DATA SUMMARY AVERAGE LENGTH TOTAL AREA NO AREA NAME FEET INCHES HARVEST 101 LAKE PIERCE 7 9.8 12 102 LAKE MARIAN 9 9.3 30 104 LAKE HATCHINEHA 8 7.9 36 105 KISSIMMEE RIVER (POOL A) 7 6.7 17 106 KISSIMMEE RIVER (POOL C) 8 8.3 17 109 LAKE ISTOKPOGA 8 0.5 116 110 LAKE KISSIMMEE 7 11.5 172 112 TENEROC FMA 8 6.0 1 402 EVERGLADES WMA (WCAs 2A & 2B) 8 8.2 12 404 EVERGLADES WMA (WCAs 3A & 3B) 8 10.4 63 405 HOLEY LAND WMA 9 11.0 2 500 BLUE CYPRESS LAKE 8 5.6 31 501 ST. JOHNS RIVER 1 8 2.2 69 502 ST. JOHNS RIVER 2 8 0.7 152 504 ST. JOHNS RIVER 4 8 3.6 83 505 LAKE HARNEY 7 8.7 65 506 ST. JOHNS RIVER 5 9 2.2 38 508 CRESCENT LAKE 8 9.9 23 510 LAKE JESUP 9 9.5 28 518 LAKE ROUSSEAU 7 9.3 32 520 LAKE TOHOPEKALIGA 9 7.1 47 547 GUANA RIVER WMA 9 4.6 5 548 OCALA WMA 9 8.7 4 549 THREE LAKES WMA 9 9.3 4 601 LAKE OKEECHOBEE (WEST) 8 11.7 448 602 LAKE OKEECHOBEE (NORTH) 9 1.8 163 603 LAKE OKEECHOBEE (EAST) 8 6.8 38 604 LAKE OKEECHOBEE (SOUTH) 8 5.2 323 711 LAKE HANCOCK 9 3.9 101 721 RODMAN RESERVOIR 8 7.0 118 722 ORANGE LAKE 8 9.3 125 723 LOCHLOOSA LAKE 9 3.4 56 734 LAKE SEMINOLE 9 1.5 16 741 LAKE TRAFFORD -

The Shellcracker
the Shellcracker FLORIDA CHAPTER OF THE AMERICAN FISHERIES SOCIETY http://www.sdafs.org/flafs April, 2011 President’s Message: Greetings from South Florida, folks. For those of you who weren’t able to attend in January, you missed one heck of a Southern Division meeting in Tampa. With 408 attendees and over 200 individual oral and presentations, we’re still getting complements on how well it turned out. I appreciate all of the help that folks around the chapter offered, but special thanks need to be given to Eric Nagid, Linda Lombardi-Carlson, Kerry Flaherty, Andy Strickland, Wes Porak, and the rest of the planning committee for their tireless efforts to make this meeting a success. As many of you know, or perhaps are fast learning, this is a challenging time to be involved in fisheries sci- ence. Federal and state employees are both facing such issues as departmental hiring freezes, field and travel budget reductions, and an increasing individual workload. On the academic side, many colleges and universities are choosing to not back-fill positions upon retirements and are similarly increasing individual workloads on remaining faculty, not to mention the general decrease in external grant and contract funding that served in the past to support many of us and our research in graduate school. We may find our work personally fulfilling, and we often get to see things during our careers that would make many people envious, but I wish sometimes I knew where the public got its perception of the cushy life of the fisheries biologist! Now, it’s one thing to complain and yet another to offer solutions, as many of us likely heard from our parents growing up. -

Blue-Green Algal Bloom Weekly Update Reporting July 2 - July 9, 2020
BLUE-GREEN ALGAL BLOOM WEEKLY UPDATE REPORTING JULY 2 - JULY 9, 2020 SUMMARY There were 37 reported site visits in the past eight days (7/2-7/9), with 36 samples collected. Algal bloom conditions were observed by the samplers at 17 sites. Satellite imagery from 7/7 shows bloom potential in Lake Okeechobee on approximately 60% coverage on the north-northeastern portion of the lake, while visible portions of the Caloosahatchee and St. Lucie rivers and estuaries in the 7/6 imagery show no observable bloom activity. Satellite imagery from 7/6 for the St. Johns River is partially obscured by cloud cover but shows minimal bloom potential in visible portions of Lake George or on the mainstem of the St. Johns River downstream of Lake George. Please keep in mind that bloom potential is subject to change due to rapidly changing environmental conditions or satellite inconsistencies (i.e., wind, rain, temperature or stage). On 7/6-7/8, South Florida Water Management District staff performed routine monitoring on Lake Okeechobee. They observed algal bloom conditions at 14 of the 28 stations they visited (NES191, NES135, EASTSHORE, L004, L008, POLESOUT2, POLESOUT3, PELBAY3, L006, L007, LZ30, PALMOUT3, LZ40 and CULV10A). Most of the stations where bloom conditions were observed were dominated by Microcystis aeruginosa, while stations without bloom conditions observed were dominated by either Microcystis aeruginosa, Cylindrospermosis raciborskii, co-dominated by Cylindrospermosis raciborskii and Planktolyngbya limnetica, or had no dominant algal taxon. Stations with detectable levels of total microcystin included: LZ2 (trace 0.31 ppb); NES191 (8.8 ppb); NES135 (3.0 ppb); EASTSHORE (7.5 ppb); L004 (17 ppb); L008 (6.0 ppb); POLESOUT3 (4.9 ppb); POLESOUT2 (3.8 ppb); L006 (1.0 ppb); LZ40 (6.2 ppb); and CVL10A (14 ppb). -

Babcock Ranch
Florida SPRING 2006 Naturalist AOF THANKS Everglades Foundation Chapter Initiatives RECOGNIZED PRESERVING Private Lands 2006 Board of Directors EXECTIVE DIRECTOR’S MESSAGE John F. Flanigan, Esq., Chairman Solving today's environmental challenges is complex. We must find a balance Moyle, Flanigan, Katz, Raymond & Sheehan between the pressures of explosive growth and the need for environmental sustainability. Doug Pitts, Sr., Vice Chair We must restore unique ecosystems and habitats that birds and wildlife depend upon. Courtelis Company Joyce King, Secretary And we must acquire natural areas to ensure their protection in perpetuity. St. Petersburg Audubon Society Audubon of Florida could not address these J.T. Ambrozy, Treasurer challenges without support from and the work of Philanthropist our strategic partners. In February, I was pleased Carole A. Adams to present Senator Bob Smith, President of the Duval County Audubon Everglades Foundation, with the fruit of an Sandy Batchelor Batchelor Enterprises important labor, the Lake Okeechobee Report. William O. Birchfield, Esq. The Report, like many of our accomplishments Birchfield & Humphrey PA in restoring the Everglades, is possible because Julie Byrne of funding from the Everglades Foundation. Sarasota Audubon Society Support also came from a variety of foundations, Ernie Caldwell Waterfront Management Services, Inc. in particular, the Batchelor Foundation, which Peggy Cox has been most generous in recent years. Orange County Audubon Society The Lake Okeechobee Report is an in-depth, Henry Dean scientific assessment of this crucial ecosystem, Former Executive Director researched and written by Dr. Paul Gray, Chris South Florida Water Management District "Restoration and protection of Everglades ecosystems Farrell, Dr. Mark Kraus and April Gromnicki. -
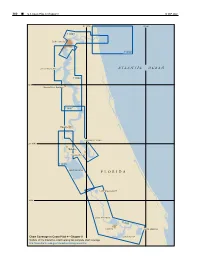
F L O R I D a Atlantic Ocean
300 ¢ U.S. Coast Pilot 4, Chapter 9 19 SEP 2021 81°30'W 81°W 11491 Jacksonville 11490 DOCTORS LAKE ATL ANTIC OCEAN 11492 30°N Green Cove Springs 11487 Palatka CRESCENT LAKE 29°30'N Welaka Crescent City 11495 LAKE GEORGE FLORIDA LAKE WOODRUFF 29°N LAKE MONROE 11498 Sanford LAKE HARNEY Chart Coverage in Coast Pilot 4—Chapter 9 LAKE JESUP NOAA’s Online Interactive Chart Catalog has complete chart coverage http://www.charts.noaa.gov/InteractiveCatalog/nrnc.shtml 19 SEP 2021 U.S. Coast Pilot 4, Chapter 9 ¢ 301 St. Johns River (1) (8) ENCs - US5FL51M, US5FL57M, US5FL52M, US- Fish havens 5FL53M, US5FL84M, US5FL54M, US5FL56M (9) Numerous fish havens are eastward of the entrance to Charts - 11490, 11491, 11492, 11487, 11495, St. Johns River; the outermost is about 31 miles eastward 11498 of St. Johns Light. (10) (2) St. Johns River, the largest in eastern Florida, is Prominent features about 248 miles long and is an unusual major river in (11) St. Johns Light (30°23'10"N., 81°23'53"W.), 83 that it flows from south to north over most of its length. feet above the water, is shown from a white square tower It rises in the St. Johns Marshes near the Atlantic coast on the beach about 1 mile south of St. Johns River north below latitude 28°00'N., flows in a northerly direction jetty. A tower at Jacksonville Beach is prominent off and empties into the sea north of St. Johns River Light in the entrance, and water tanks are prominent along the latitude 30°24'N. -

Kissimmee Chain of Lakes Long-Term Management Plan Project
22001111 IInntteerraaggeennccyy DDrraafftt KKiissssiimmmmeeee CChhaaiinn ooff LLaakkeess LLoonngg--TTeerrmm MMaannaaggeemmeenntt PPllaann South Florida Water Management District Florida Fish and Wildlife Conservation Commission Florida Department of Environmental Protection Florida Department of Agriculture and Consumer Services U.S. Army Corps of Engineers U.S. Fish and Wildlife Service Osceola County June 2011 Acknowledgments The South Florida Water Management District (SFWMD) would like to acknowledge the partner agencies and local governments and their staffs for their participation and commitment to the Kissimmee Chain of Lakes Long-Term Management Plan project. This dedicated team of professionals has exercised due diligence in the compilation, preparation, and review of the materials presented within this document and has worked to define and embrace a common vision of health for the Kissimmee Chain of Lakes resource. The SFWMD also acknowledges all the other stakeholders who have provided valuable insights into and perspectives on the value of the resource and the need for federal, state, and local government agencies to work together to preserve and protect the natural resources of central Florida. For further information about this document, please contact: Chris Carlson South Florida Water Management District 3301 Gun Club Road West Palm Beach, FL 33406 Telephone: (561) 682-6143 Email: [email protected] Interagency Team Draft Revision 2011 CONTENTS ACRONYMS AND ABBREVIATIONS ...................................................................................... -

Your Guide to Eating Fish Caught in Florida
Fish Consumption Advisories are published periodically by the Your Guide State of Florida to alert consumers about the possibility of chemically contaminated fish in Florida waters. To Eating The advisories are meant to inform the public of potential health risks of specific fish species from specific Fish Caught water bodies. In Florida Florida Department of Health Prepared in cooperation with the Florida Department of Environmental Protection and Agriculture and Consumer Services, and the Florida Fish and Wildlife Conservation Commission 2016 Florida Fish Advisories • Table 1: Eating Guidelines for Fresh Water Fish from Florida Waters page 1-33 • Table 2: Eating Guidelines for Marine and Estuarine Fish from Florida Waters page 34-35 • Table 3: Eating Guidelines for species from Florida Waters with Heavy Metals, Dioxin, Pesticides, Polychlorinated biphenyls (PCBs), or Saxitoxin Contamination page 36 Eating Fish is an important part of a healthy diet. Rich in vitamins and low in fat, fish contains protein we need for strong bodies. It is also an excellent source of nutrition for proper growth and development. In fact, the American Heart Association recommends that you eat two meals of fish or seafood every week. At the same time, most Florida seafood has low to medium levels of mercury. Depending on the age of the fish, the type of fish, and the condition of the water the fish lives in, the levels of mercury found in fish are different. While mercury in rivers, creeks, ponds, and lakes can build up in some fish to levels that can be harmful, most fish caught in Florida can be eaten without harm.