Haemogenic Endocardium Contributes to Transient Definitive Haematopoiesis
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

Chapter Xi the Circulatory System and Blood
CHAPTER XI THE CIRCULATORY SYSTEM AND BLOOD Page General characterlstlcs______ __ __ _ __ __ __ __ __ __ _ 239 of these organs are independent of the beating of The pericardium ___ __ __ __ 239 the principal heart, and their primary function is The heart. _____ __ __ 240 Physiology of the heart.______________________________________________ 242 to oscillate the blood within the pallial sinuses. Automatism of heart beat. _ 242 The pacemaker system_ 245 THE PERICARDIUM Methods of study of heart beat_____________________________________ 247 Frequency of beat___ __ __ _ 248 Extracardlac regulatlon____ __ __ _ 250 The heart is located in the pericardium, a thin Effects of mineral salts and drugs___________________________________ 251 Blood vessels_ __ ___ _ 253 walled chamber between the visceral mass and the The arterial system______ __ __ ___ __ __ __ __ __ 253 adductor muscle (fig. 71). In a live oyster the The venous system_________________________________________________ 254 location of the heart is indicated by the throbbing The accessory heart._____________ 258 The blood______ __ __ __ __ __ __ __ 259 of the wall of the pericardium on the left side. Color of blood_ __ __ 261 Here the pericardium wall lies directly under the The hyaline cells___________________________________________________ 261 The granular cells .______________________________________ 262 shell. On the right side the promyal chamber Specific gravity of blood____________________________________________ 265 extends down over the heart region and the mantle Serology ___ __________ __________________ ____ __ ______________________ 265 Bibliography __ __ __ __ __ __ __ 266 separates the pericardium wall from the shell. The cavity in which the heart is lodged is slightly A heart, arteries, veins, and open sinuses form asymmetrical; on the right side it extends farther the circulatory system of oysters and other bi along the anterior part of the adductor muscle valves. -

Download PDF File
Folia Morphol. Vol. 78, No. 2, pp. 283–289 DOI: 10.5603/FM.a2018.0077 O R I G I N A L A R T I C L E Copyright © 2019 Via Medica ISSN 0015–5659 journals.viamedica.pl Observations of foetal heart veins draining directly into the left and right atria J.H. Kim1, O.H. Chai1, C.H. Song1, Z.W. Jin2, G. Murakami3, H. Abe4 1Department of Anatomy and Institute of Medical Sciences, Chonbuk National University Medical School, Jeonju, Korea 2Department of Anatomy, Wuxi Medical School, Jiangnan University, Wuxi, China 3Division of Internal Medicine, Jikou-kai Clinic of Home Visits, Sapporo, Japan 4Department of Anatomy, Akita University School of Medicine, Akita, Japan [Received: 19 June 2018; Accepted: 8 August 2018] Evaluation of semiserial sections of 14 normal hearts from human foetuses of gestational age 25–33 weeks showed that all of these hearts contained thin veins draining directly into the atria (maximum, 10 veins per heart). Of the 75 veins in these 14 hearts, 55 emptied into the right atrium and 20 into the left atrium. These veins were not accompanied by nerves, in contrast to tributaries of the great cardiac vein, and were negative for both smooth muscle actin (SMA) and CD34. However, the epithelium and venous wall of the anterior cardiac vein, the thickest of the direct draining veins, were strongly positive for SMA and CD34, respectively. In general, developing fibres in the vascular wall were positive for CD34, while the endothelium of the arteries and veins was strongly positive for the present DAKO antibody of SMA. -

Location of the Human Sinus Node in Black Africans
ogy: iol Cu ys r h re P n t & R y e s Anatomy & Physiology: Current m e o a t r a c n h A Research Meneas et al., Anat Physiol 2017, 7:5 ISSN: 2161-0940 DOI: 10.4172/2161-0940.1000279 Research article Open Access Location of the Human Sinus Node in Black Africans Meneas GC*, Yangni-Angate KH, Abro S and Adoubi KA Department of Cardiovascular and Thoracic Diseases, Bouake Teaching Hospital, Cote d’Ivoire, West-Africa *Corresponding author: Meneas GC, Department of Cardiovascular and Thoracic Diseases, Bouake Teaching Hospital, Cote d’Ivoire, West-Africa, Tel: +22507701532; E-mail: [email protected] Received Date: August 15, 2017; Accepted Date: August 22, 2017; Published Date: August 29, 2017 Copyright: © 2017 Meneas GC, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution and reproduction in any medium, provided the original author and source are credited. Abstract Objective: The purpose of this study was to describe, in 45 normal hearts of black Africans adults, the location of the sinoatrial node. Methods: After naked eye observation of the external epicardial area of the sinus node classically described as cavoatrial junction (CAJ), a histological study of the sinus node area was performed. Results: This study concluded that the sinus node is indistinguishable to the naked eye (97.77% of cases), but still identified histologically at the CAJ in the form of a cluster of nodal cells surrounded by abundant connective tissues. It is distinguished from the Myocardial Tissue. -

4B. the Heart (Cor) 1
Henry Gray (1821–1865). Anatomy of the Human Body. 1918. 4b. The Heart (Cor) 1 The heart is a hollow muscular organ of a somewhat conical form; it lies between the lungs in the middle mediastinum and is enclosed in the pericardium (Fig. 490). It is placed obliquely in the chest behind the body of the sternum and adjoining parts of the rib cartilages, and projects farther into the left than into the right half of the thoracic cavity, so that about one-third of it is situated on the right and two-thirds on the left of the median plane. Size.—The heart, in the adult, measures about 12 cm. in length, 8 to 9 cm. in breadth at the 2 broadest part, and 6 cm. in thickness. Its weight, in the male, varies from 280 to 340 grams; in the female, from 230 to 280 grams. The heart continues to increase in weight and size up to an advanced period of life; this increase is more marked in men than in women. Component Parts.—As has already been stated (page 497), the heart is subdivided by 3 septa into right and left halves, and a constriction subdivides each half of the organ into two cavities, the upper cavity being called the atrium, the lower the ventricle. The heart therefore consists of four chambers, viz., right and left atria, and right and left ventricles. The division of the heart into four cavities is indicated on its surface by grooves. The atria 4 are separated from the ventricles by the coronary sulcus (auriculoventricular groove); this contains the trunks of the nutrient vessels of the heart, and is deficient in front, where it is crossed by the root of the pulmonary artery. -

Sudden Death in Racehorses: Postmortem Examination Protocol
VDIXXX10.1177/1040638716687004Sudden death in racehorsesDiab et al. 687004research-article2017 Special Issue Journal of Veterinary Diagnostic Investigation 1 –8 Sudden death in racehorses: postmortem © 2017 The Author(s) Reprints and permissions: sagepub.com/journalsPermissions.nav examination protocol DOI: 10.1177/1040638716687004 jvdi.sagepub.com Santiago S. Diab,1 Robert Poppenga, Francisco A. Uzal Abstract. In racehorses, sudden death (SD) associated with exercise poses a serious risk to jockeys and adversely affects racehorse welfare and the public perception of horse racing. In a majority of cases of exercise-associated sudden death (EASD), there are no gross lesions to explain the cause of death, and an examination of the cardiovascular system and a toxicologic screen are warranted. Cases of EASD without gross lesions are often presumed to be sudden cardiac deaths (SCD). We describe an equine SD autopsy protocol, with emphasis on histologic examination of the heart (“cardiac histology protocol”) and a description of the toxicologic screen performed in racehorses in California. By consistently utilizing this standardized autopsy and cardiac histology protocol, the results and conclusions from postmortem examinations will be easier to compare within and across institutions over time. The generation of consistent, reliable, and comparable multi-institutional data is essential to improving the understanding of the cause(s) and pathogenesis of equine SD, including EASD and SCD. Key words: Cardiac autopsy; equine; exercise; racehorses; -

Ultrastructure of the Normal Atrial Endocardium R
Brit. Heart J., 1966, 28, 785. Ultrastructure of the Normal Atrial Endocardium R. A. LANNIGAN AND SALEH A. ZAKI From the Department of Pathology, University of Birmingham, and the Department of Pathology, University of Assiut, U.A.R. Surgical biopsies of the heart and early needle Thin sections were then cut and examined on an A.E.I. "biopsies" at necropsy now permit ultrastructural E M 6 electron microscope. investigations into many cardiac conditions. There For light microscopy, blocks of the whole circum- are, however, very few such studies on normal human ference were embedded in paraffin and sections were stained with Ehrlich's hTmatoxylin and eosin, van myocardium and none are available on human or Gieson's stain, Lawson's modification of the Weigert- animal endocardium. The commonest surgical Sheridan elastic stain, the periodic-acid Schiff reaction, specimens from the heart are from the left or right and Hale's dialysed iron. atrial appendages, and this material has been used to study normal structure, as a baseline for the investigation of cardiac diseases such as rheumatic RESULTS heart disease and fibro-elastosis, etc. For obvious Light Microscopy reasons, it is difficult to obtain fresh material from "normal" hearts, and specimens were selected for The structure of the left atrial wall has been electron microscopy which were within normal described by Koniger (1903), Nagayo (1909), Von limits on the light microscope (Lannigan, 1959). Glahn (1926), and Gross (1935), and the structure of the atrial appendages, which was shown to be similar to that of the atrium, was described by MATERIAL AND METHODS Waaler (1952), Lannigan (1959), and Ling, Wang, Three left atrial appendages and three right append- and Kuo (1959). -

Abnormally Enlarged Singular Thebesian Vein in Right Atrium
Open Access Case Report DOI: 10.7759/cureus.16300 Abnormally Enlarged Singular Thebesian Vein in Right Atrium Dilip Kumar 1 , Amit Malviya 2 , Bishwajeet Saikia 3 , Bhupen Barman 4 , Anunay Gupta 5 1. Cardiology, Medica Institute of Cardiac Sciences, Kolkata, IND 2. Cardiology, North Eastern Indira Gandhi Regional Institute of Health and Medical Sciences, Shillong, IND 3. Anatomy, North Eastern Indira Gandhi Regional Institute of Health and Medical Sciences, Shillong, IND 4. Internal Medicine, North Eastern Indira Gandhi Regional Institute of Health and Medical Sciences, Shillong, IND 5. Cardiology, Vardhman Mahavir Medical College (VMMC) and Safdarjung Hospital, New Delhi, IND Corresponding author: Amit Malviya, [email protected] Abstract Thebesian veins in the heart are subendocardial venoluminal channels and are usually less than 0.5 mm in diameter. The system of TV either opens a venous (venoluminal) or an arterial (arterioluminal) channel directly into the lumen of the cardiac chambers or via some intervening spaces (venosinusoidal/ arteriosinusoidal) termed as sinusoids. Enlarged thebesian veins are reported in patients with congenital heart disease and usually, multiple veins are enlarged. Very few reports of such abnormal enlargement are there in the absence of congenital heart disease, but in all such cases, they are multiple and in association with coronary artery microfistule. We report a very rare case of a singular thebesian vein in the right atrium, which was abnormally enlarged. It is important to recognize because it can be confused with other cardiac structures like coronary sinus during diagnostic or therapeutic catheterization and can lead to cardiac injury and complications if it is attempted to cannulate it or pass the guidewires. -
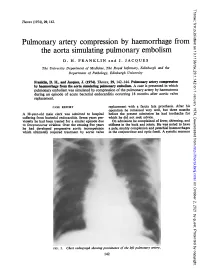
The Aorta Simulating Pulmonary Embolism D
Thorax: first published as 10.1136/thx.29.1.142 on 1 January 1974. Downloaded from Thorax (1974), 29, 142. Pulmonary artery compression by haemorrhage from the aorta simulating pulmonary embolism D. H. FRANKLIN and J. JACQUES The University Department of Medicine, The Royal Infirmary, Edinburgh and the Department of Pathology, Edinburgh University Franklin, D. H., and Jacques, J. (1974). Thorax, 29, 142-144. Pulmonary artery compression by haemorrhage from the aorta simulating pulmonary embolism. A case is presented in which pulmonary embolism was simulated by compression of the pulmonary artery by haematoma during an episode of acute bacterial endocarditis occurring 18 months after aortic valve replacement. CASE REPORT replacement with a fascia lata prosthesis. After his operation he remained very well, but three months A 38-year-old male clerk was admitted to hospital before the present admission he had toothache for suffering from bacterial efndocarditis. Seven years pre- which he did not seek advice. viomly he had been treated for a similar episode due On admission he complained of fever, shivering, and to Streptococcus viridans. Over the ensuing five years stiffness in the back and joints. He was noted to have he had developed progressive aortic incompetence a pale, muddy complexion and petechial haemorrhages which ultimately required treatment by aortic valve in the conjunctivae and optic fundi. A systolic murmur http://thorax.bmj.com/ on October 2, 2021 by guest. Protected copyright. FIG. 1. Chesvt radiograph showing prominence of the left pulmonary artery. 142 Thorax: first published as 10.1136/thx.29.1.142 on 1 January 1974. -

The American Society of Echocardiography
1 THE AMERICAN SOCIETY OF ECHOCARDIOGRAPHY RECOMMENDATIONS FOR CARDIAC CHAMBER QUANTIFICATION IN ADULTS: A QUICK REFERENCE GUIDE FROM THE ASE WORKFLOW AND LAB MANAGEMENT TASK FORCE Accurate and reproducible assessment of cardiac chamber size and function is essential for clinical care. A standardized methodology creates a common approach to the assessment of cardiac structure and function both within and between echocardiography labs. This facilitates better communication and improves the ability to compare results between studies as well as differentiate normal from abnormal findings in an individual patient. This document summarizes key points from the 2015 ASE Chamber Quantification Guideline and is meant to serve as quick reference for sonographers and interpreting physicians. It is designed to provide guidance on chamber quantification for adult patients; a separate ASE Guidelines document that details recommended quantification methods in the pediatric age group has also been published and should be used for patients <18 years of age (3). (1) For details of the methodology and the rationale for current recommendations, the interested reader is referred to the complete Guideline statement. Figures and tables are reproduced from ASE Guidelines. (1,2) Table of Contents: 1. Left Ventricle (LV) Size and Function p. 2 a. LV Size p. 2 i. Linear Measurements p. 2 ii. Volume Measurements p. 2 iii. LV Mass Calculations p. 3 b. Left Ventricular Function Assessment p. 4 i. Global Systolic Function Parameters p. 4 ii. Regional Function p. 5 2. Right Ventricle (RV) Size and Function p. 6 a. RV Size p. 6 b. RV Function p. 8 3. Atria p. -

Structures of the Heart
Anatomy Tip Structures of the Heart In an effort to aid Health Information Management Coding Professionals for ICD-10, the following anatomy tip is provided with an educational intent. TIP: The Heart is made up of three main structures: 1. The Pericardium 2. The Heart Wall 3. The Chambers of the Heart The pericardium surrounds the heart. The outer layer, called the fibrous pericardium, secures the heart to surrounding structures like the blood vessels and the diaphragm. The inner layer, called the serous pericardium, is a double-layered section of the heart. Inside the two layers, serous fluid, known as pericardial fluid, lubricates and helps the heart move fluidly when beating. The heart wall is made up of three tissue layers: the epicardium, the myocardium, and the endocardium. The epicardium, which is the outermost layer, is also known as the visceral pericardium because it is also the inner wall of the pericardium. The middle layer, known as the myocardium, is formed out of contracting muscle. The endocardium, the innermost layer, covers heart valves and acts as a lining of the heart chambers. It is also in contact with the blood that is pumped through the heart, in order to push blood into the lungs and throughout the rest of the body. The four heart chambers are crucial to the heart’s function, composed of the atria, the right atrium and left atrium, and ventricles, the right ventricle and left ventricle. The right and left atria are thin-walled chambers responsible for receiving blood from veins, while the left and right ventricle are thick-walled chambers responsible for pumping blood out of the heart. -

Premature Obliteration of the Foramen Ovale by G
PREMATURE OBLITERATION OF THE FORAMEN OVALE BY G. AUSTIN GRESHAM From the Department of Pathology, University of Cambridge Received August 16, 1955 Obliteration of the foramen ovale occurring during intrauterine life is a rare condition. It throws some light on the mechanism of development of endocardial fibroelastosis and also upon the factors concerned in determining the size of the aorta and of the cardiac chambers. CASE HISTORY The patient was the second child of a mother (aet. 21) whose previous obstetric history was normal. Apart from two periods of rather rapid gain of weight for which no cause could be found, the pregnancy was uneventful. The child was eleven days postmature; labour was induced with an enema and lasted forty-five minutes. The infant cried lustily but was cyanosed: the heart was clinically normal. Abnormalities were present in all four limbs. The left radius and ulna were absent and rudimentary digits were present on the skin over the distal end of the limb. Terminal phalanges were absent in the fingers of the right hand, and four toes were present on each foot with a rudimentary fifth digit on the left foot. Cyanosis and dyspniea became more intense and the child died three hours after birth despite the use of oxygen. NECROPSY The body was that of a full-term male infant (weight 3430 g.). The limbs were abnormal as previously described. The lips were blue-black in colour. On the septal wall of the right atrium a hemispherical grey-white area (10x 12 x 4 mm. deep) with a central dimple filled in the usual site of the fossa ovalis (Fig. -

The Cardiovascular System
11 The Cardiovascular System WHAT The cardiovascular system delivers oxygen and HOW nutrients to the body tissues The heart pumps and carries away wastes blood throughout the body such as carbon dioxide in blood vessels. Blood flow via blood. requires both the pumping action of the heart and changes in blood pressure. WHY If the cardiovascular system cannot perform its functions, wastes build up in tissues. INSTRUCTORS Body organs fail to function properly, New Building Vocabulary and then, once oxygen becomes Coaching Activities for this depleted, they will die. chapter are assignable in hen most people hear the term cardio- only with the interstitial fluid in their immediate Wvascular system, they immediately think vicinity. Thus, some means of changing and of the heart. We have all felt our own “refreshing” these fluids is necessary to renew the heart “pound” from time to time when we are ner- nutrients and prevent pollution caused by vous. The crucial importance of the heart has been the buildup of wastes. Like a bustling factory, the recognized for ages. However, the cardiovascular body must have a transportation system to carry system is much more than just the heart, and its various “cargoes” back and forth. Instead of from a scientific and medical standpoint, it is roads, railway tracks, and subways, the body’s important to understand why this system is so vital delivery routes are its hollow blood vessels. to life. Most simply stated, the major function of the Night and day, minute after minute, our tril- cardiovascular system is transportation. Using lions of cells take up nutrients and excrete wastes.