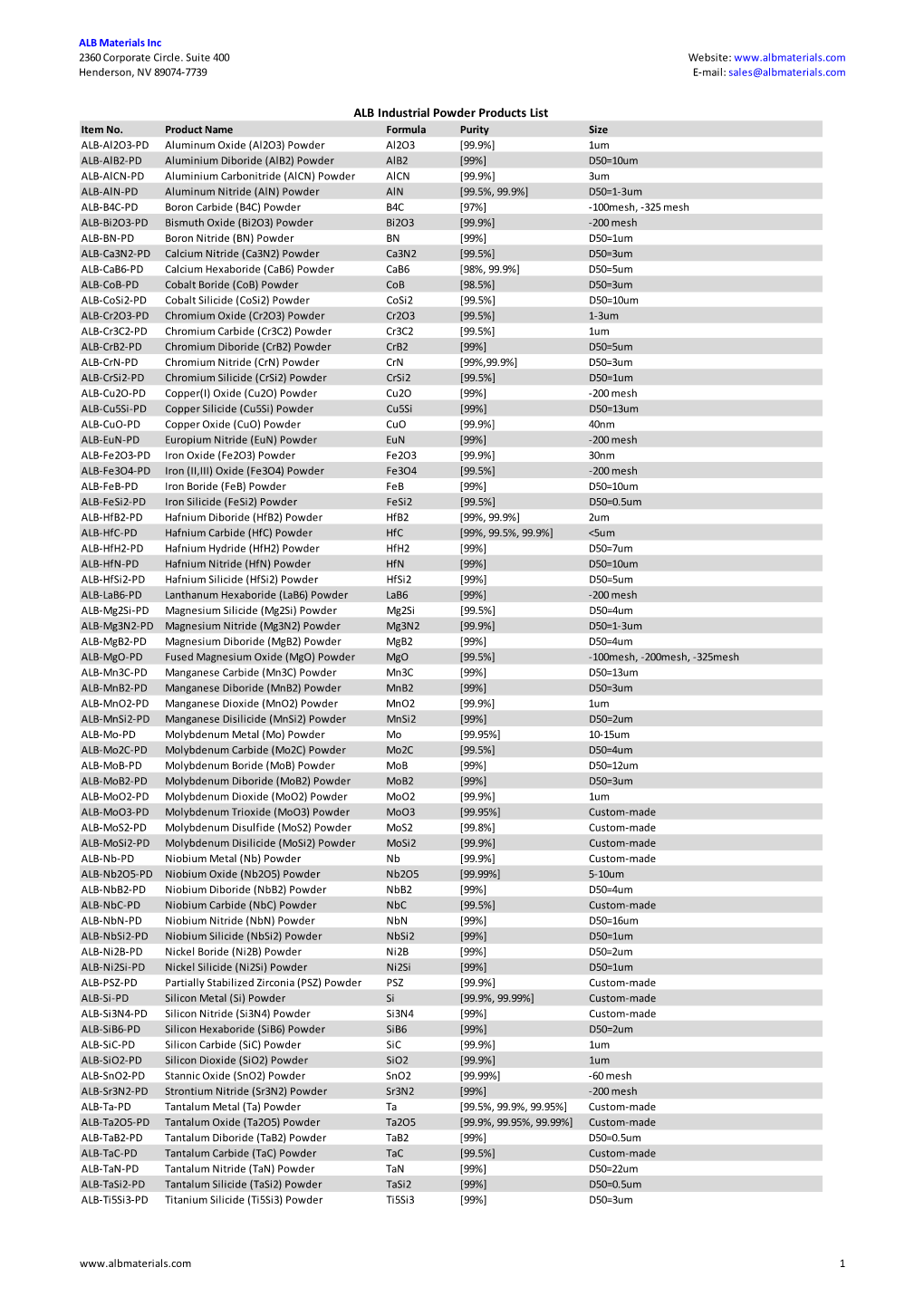
ALB Materials Inc Product List.Xlsx
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

LOUISIANA SCIENTIST Vol. 1A No. 3
LOUISIANA SCIENTIST THE NEWSLETTER of the LOUISIANA ACADEMY OF SCIENCES Volume 1A, No. 3 (2007 Annual Meeting Abstracts) Published by THE LOUISIANA ACADEMY OF SCIENCES 15 June 2012 Louisiana Academy of Sciences Abstracts of Presentations 2007 Annual Meeting Southern University and A&M College Baton Rouge, Louisiana 16 March 2007 Table of Contents Division/Section Page Division of Agriculture, Forestry, and Wildlife . 5 Division of Biological Sciences . 11 Botany Section . 11 Environmental Sciences Section . 11 Microbiology Section . 17 Molecular and Biomedical Biology Section . 21 Zoology Section . 23 Division of Physical Sciences . 28 Chemistry Section . 28 Computer Science Section . 34 Earth Sciences Section . 41 Materials Science and Engineering Section . 43 Mathematics and Statistics Section . 46 Physics Section . 49 Division of Science Education . 52 Higher Education Section . 52 K-12 Education Section . 55 Division of Social Sciences . 57 Acknowledgement . 64 2 The following abstracts of oral and poster presentations represent those received by the Abstract Editor. Authors’ affiliations are abbreviated as follows: ACHRI Arkansas Children’s Hospital Research Institute ARS Agriculture Research Services, Little Rock, AR AVMA-PLIT American Veterinary Medical BGSU Bowling Green State University BNL Brookhaven National Laboratory, Upton, NY BRCC Baton Rouge Community College CC Centenary College CIT California Institute of Technology CL Corrigan Laboratory, Baton Rouge, LA CTF Cora Texas Manufacturing CU Clemson University DNIRI Delta -

Growth and Characterization of Bn Thin Films Deposited by Pacvd
GROWTH AND CHARACTERIZATION OF BN THIN FILMS DEPOSITED BY PACVD A thesis for the degree of Ph.D. Presented to DUBLIN CITY UNIVERSITY by MD. ZIAUL KARIM, M. Sc.(EEE) SCHOOL OF ELECTRONIC ENGINEERING DUBLIN CITY UNIVERSITY RESEARCH SUPERVISOR DR. DAVID C. CAMERON February, 1993. TO THE DEAREST MEMBERS OF SHAPBNIL LODGE MUM, DAD PIAS, LUCKY, SOHAIL & BIPLAB DECLARATION I hereby certify that this material, which I now submit for the assessment on the programme of study leading to the award of Ph.D. is entirely my own work and has not been taken from the work of others save and to the extent that such work has been cited and acknowledged within the text of my work. Signed: Date: February 2, 1993. ACKNOWLEDGEMENTS I am greatly indebted to Dr. David Cameron for his guidance and tuition in the course of this work. Without his enthusiasm and continual drive to increase our understanding of plasma technology, we would not have acquired such an in-depth knowledge of plasma techniques. To Professor M. S. J. Hashmi, a special word of appreciation and gratitude for his guidance and enthusiasm and for affording me the opportunity to pursue this work. I must also acknowledge the help of my fellow Mend, Michael Murphy for his continuous cooperation throughout the last few years and for helping me in writing this thesis. Without the continued assistance of both Hafizur Rahman and Rowshan Hafiz in preparing this thesis, it would not have been possible to complete it for me in the present time scale just after my glandular fever. -

1. Give the Correct Names for Each of the Compounds Listed Below. A
1. Give the correct names for each of the compounds listed below. a) NaCl sodium chloride n) ZrS2 zirconium sulfide b) FrBr francium bromide o) AgI silver iodide c) KF potassium fluoride p) BaSe barium selenide d) RaS radium sulfide q) MgO magnesium oxide e) LiI lithium iodide r) LaBr3 lanthanum bromide f) Li3N lithium nitride s) Sr3N2 strontium nitride g) AlBr3 aluminum bromide t) Cd3As2 cadmium arsenide h) CdCl2 cadmium chloride u) Rb2Se rubidium selenide i) K2O potassium oxide v) Rb3N rubidium nitride j) InF3 indium fluoride w) BaF2 barium fluoride k) ZnO zinc oxide x) ZrTe2 zirconium telluride l) Y2O3 yttrium oxide y) Cs3P cesium phosphide m) CaTe calcium telluride z) Y2O3 yttrium oxide 2. Write the correct chemical formula for each of the following compounds. a) potassium bromide KBr n) potassium nitride K3N b) zinc bromide ZnBr2 o) aluminum bromide AlBr3 c) lithium iodide LiI p) zinc phosphide Zn3P2 d) scandium chloride ScCl3 q) magnesium sulfide MgS e) magnesium chloride MgCl2 r) hafnium chloride HfCl4 f) magnesium oxide MgO s) barium sulfide BaS g) hydrogen sulfide H2S t) tantalum oxide Ta2O5 h) gallium iodide GaI3 u) zirconium nitride Zr3N4 i) sodium oxide Na2O v) potassium selenide K2Se j) magnesium selenide MgSe w) germanium fluoride GeF4 k) calcium fluoride CaF2 x) francium phosphide Fr3P l) aluminum oxide Al2O3 y) zinc arsenide Zn3As2 m) beryllium chloride BeCl2 z) scandium telluride Sc2Te3 L. h. s. – Chemistry – Nomenclature – Answers – Page 1 3. Give the correct names for each of the compounds listed below. a) CaSO4 calcium -

Single-Crystal Calcium Hexaboride Nanowires: Synthesis And
NANO LETTERS 2004 Single-Crystal Calcium Hexaboride Vol. 4, No. 10 Nanowires: Synthesis and 2051-2055 Characterization Terry T. Xu,† Jian-Guo Zheng,‡ Alan W. Nicholls,§ Sasha Stankovich,† Richard D. Piner,† and Rodney S. Ruoff*,† Department of Mechanical Engineering, Northwestern UniVersity, EVanston, Illinois 60208, NUANCE Center, Northwestern UniVersity, EVanston, Illinois 60208, and Research Resource Center, UniVersity of Illinois at Chicago, Chicago, Illinois 60612 Received August 17, 2004 ABSTRACT Catalyst-assisted growth of single-crystal calcium hexaboride (CaB6) nanowires was achieved by pyrolysis of diborane (B2H6) over calcium oxide (CaO) powders at 860−900 °C and ∼155 mTorr in a quartz tube furnace. TEM electron diffraction and Raman spectroscopy indicate that the nanowires are single-crystal CaB6 and have a preferred [001] growth direction. Analysis of TEM/EDX/EELS data proves the nanowires consist of CaB6 cores and thin (1−2 nm) amorphous oxide shell material. The CaB6 nanowires have diameters of ∼15−40 nm, and lengths of ∼1−10 µm. -2 22 Calcium hexaboride (CaB6), one of the divalent alkaline- (CaCO3) and boron carbide (B4C) at 1400 °C and 10 Pa, earth cubic hexaborides, has low density (2.45 g/cm3), high by reduction of calcium oxide (CaO) with B at 1200-1800 3 melting point (2235 °C), high hardness (Knoop: 2600 kg/ °C in vacuum, and by reaction of calcium chloride (CaCl2) 2 mm ), high Young’s modulus (379 GPa), and high chemical with sodium borohydride (NaBH4)at500°C in an autoclave 1,2 23 stability. CaB6 has found application as a high-temperature for8h. The finest crystalline CaB6 powders synthesized material, is used for surface protection, and as a wear resistant so far have an average size of 180 nm.23 1,2 material. -

"Boron Halides"
138 BORON HALIDES Vol. 4 BORON HALIDES 1. Introduction The boron trihalides boron trifluoride [7637-07-2], BF3, boron trichloride [10294- 34-5], BCl3, and boron tribromide [10294-33-4], BBr3, are important industrial chemicals having increased usage as Lewis acid catalysts and in chemical vapor deposition (CVD) processes (see ELECTRONIC MATERIALS). Boron halides are widely used in the laboratory as catalysts and reagents in numerous types of organic reactions and as starting material for many organoboron and inorganic boron compounds. An exhaustive review of the literature on boron halides up to 1984 is available (1–5). Of particular interest are review articles on BCl3 (1), BBr3 (2), and boron triiodide [13517-10-7], BI3 (3). An excellent review on diboron tetrahalides and polyhedral boron halides is available (6). Kirk-Othmer Encyclopedia of Chemical Technology. Copyright John Wiley & Sons, Inc. All rights reserved. Vol. 4 BORON HALIDES 139 2. Physical Properties 2 Boron trihalides, BX3, are trigonal planar molecules which are sp hybridized. The X–B–X angles are 1208. Important physical and thermochemical data are presented in Table 1 (7–13). Additional thermodynamic and spectroscopic data may be found in the literature (1–5). Table 1. Physical Properties of the Boron Trihalides References Property BF3 BCl3 BBr3 BI3 BCl3 BBr3 BI3 BF3 mp, 8C À128.37 À107 À46 À49:977714 bp, 8C À99.9 12.5 91.3 210 7 7 7 14 a : 0 : 18 density , g/mL (liq) 1 4344 2 6434 3.35 8 9 : 11 1 3494 critical temperature, À12.25 Æ 0.03 178.8 300 7 7 14 8C critical pressure, -

Low-Temperature Synthesis of Boride Powders by Controlling Microstructure in Precursor Using Organic Compounds
Journal of the Ceramic Society of Japan 126 [8] 602-608 2018 -Japan DOI http://doi.org/10.2109/jcersj2.18093 JCS SPECIAL ARTICLE The 72th CerSJ Awards for Advancements in Ceramic Science and Technology: Review Low-temperature synthesis of boride powders by controlling microstructure in precursor using organic compounds Masaki KAKIAGE1,³ 1 Institute for Fiber Engineering, Shinshu University (IFES), Interdisciplinary Cluster for Cutting Edge Research (ICCER), Shinshu University, 3–15–1 Tokida, Ueda, Nagano 386–8567, Japan The carbothermal reduction of boron oxide (B2O3) is an important process for the synthesis of boride powders. As a low-temperature synthesis method for boron carbide (B4C) powder by carbothermal reduction, we focused on an approach using a condensed product prepared from boric acid (H3BO3) and an organic compound with a number of hydroxyl groups (a polyol) such as glycerin, mannitol, or poly(vinyl alcohol). A borate ester bond was formed by the dehydration condensation of H3BO3 and a polyol, leading to the homogeneous dispersion of the boron and carbon sources at the molecular level. The thermal decomposition of a condensed H3BO3-polyol product in air was performed to control the amount of carbon to the stoichiometric C/B2O3 ratio required for carbothermal reduction. Within the thermally decomposed product consisting of B2O3 and carbon compo- nents (B4C precursor), a B2O3/carbon structure at the nanometer scale was formed. The improved dispersibility and homogeneity of the B2O3/carbon microstructure accelerated the B4C formation at a lower temperature. Consequently, crystalline B4C powder with little free carbon was synthesized by heat treatment at a low temperature of 1200°C in an Ar flow. -

Low-Temperature Synthesis of Calcium Hexaboride Nanoparticles Via Magnesiothermic Reduction in Molten Salt
Journal of the Ceramic Society of Japan 125 [12] 866-871 2017 Full paper Low-temperature synthesis of calcium hexaboride nanoparticles via magnesiothermic reduction in molten salt Ke BAO, Liangxu LIN, Hong CHANG and Shaowei ZHANG³ College of Engineering, Mathematics and Physical Sciences, University of Exeter, Exeter EX4 4QF, UK Calcium hexaboride (CaB6) nanoparticles were prepared via low temperature magnesiothermic reduction of CaO and B2O3 in molten NaCl, KCl or CaCl2. The effects of salt type, Mg amount, and firing temperature and time on the reaction extents were examined, and the responsible reaction mechanisms were discussed. Under an identical firing condition, CaCl2 facilitated the overall synthesis more effectively than the other two salts. In the case of using 20 mol % excessive Mg, phase-pure CaB6 nanoparticles of ³50 nm were formed in CaCl2 after 6 h at 800°C. The “dissolution-precipitation” mechanism is believed to be responsible for the molten salt synthesis of high quality nanosized CaB6 particles at such a low temperature. ©2017 The Ceramic Society of Japan. All rights reserved. Key-words : Calcium hexaboride, Molten salt synthesis, Reduction, Boron oxide, Calcium oxide [Received May 3, 2017; Accepted September 20, 2017] cursors. The effects of salt type, Mg amount, and firing temper- 1. Introduction ature and time on the CaB6 formation were investigated, based on Calcium hexaboride (CaB6) is a divalent alkaline-earth cubic which, the synthesis conditions optimized, and the responsible hexaboride characterized by low density (2.45 g/cm3), high melt- mechanisms underpinning proposed. ing point (2235°C), high hardness/Young’s modulus [27 GPa (Hv)/379 GPa], high electrical conductivity, as well as good 2. -

Chemsherpa 管理対象物質 Ver2.04.00 説明書
chemSHERPA 管理対象物質 Ver2.04.00 説明書 chemSHERPA 事務局 内容 1. はじめに .......................................................................................................... 2 2. 管理対象物質リストに関わる用語の定義 ..................................................................... 3 3. 管理対象物質 Ver2.04.00 について ........................................................................ 4 3.1 管理対象基準 ............................................................................................. 4 3.1.1 LR01: 日本 化審法 第一種特定化学物質 ..................................................... 5 3.1.2 LR02:米国 有害物質規制法(Toxic Substances Control Act:TSCA)使用禁止又 は制限の対象物質(第 6 条) .................................................................... 5 3.1.3 LR03:EU ELV 指令 2000/53/EC ............................................................. 6 3.1.4 LR04:EU RoHS 指令 2011/65/EU ANNEX II ........................................... 6 3.1.5 LR05:EU POPs 規則 (EU) 2019/1021 ANNEX I ....................................... 7 3.1.6 LR06 : EU REACH 規則 (EC) No 1907/2006 Candidate List of SVHC for Authorisation(認可対象候補物質)及び ANNEX XIV(認可対象物質) .............. 7 3.1.7 LR07:EU REACH 規則 (EC) No 1907/2006 ANNEX XVII(制限対象物質)... 10 3.1.8 LR08: 欧州 医療機器規則 Annex I 10.4 化学物質 ..................................... 10 3.1.9 IC01:Global Automotive Declarable Substance List (GADSL) ................... 11 3.1.10 IC02 : IEC 62474 DB Declarable substance groups and declarable substances ....................................................................................... 11 3.2 検索用物質リスト ....................................................................................... -

Answers to Problems Chapter 2 2-2 VB' B1u ; V13•14, B3u ; V I6.17.IB' B1u (B) Trap Blohl4 at - 20 to - 78°, 2-3 Diagram Very Similar to Fig
Answers to problems Chapter 2 2-2 VB' b1u ; V13•14, b3u ; V I6.17.IB' b1u (b) Trap BlOHl4 at - 20 to - 78°, 2-3 Diagram very similar to Fig. 2-12 BsH9 at -95 to -126°, B2H6 at 2-4 B1H~+ might have such a struc -196° ture, not B1H6 (c) B2H6+Me3N --+ Me3N,BH3 2-5 (a, b) IH, 1111 quartet; 11B, 1331 (in volatile) +!BZH6 quartet 4-8 MezNH,BH3; Me1NB1Hs; (c) IH, 1111 quartet, 1234321 Me2NBH2; (Me1N}zBH; septet; 11 B 1331 quartet of doub B(NMe1)3 lets 4-15 1,2-Tetramethylenediborane (d) IH, as for (c), but intensities Hydrolysis --+ 4 :1, not 6:1; 11B 121 triplet of (HO}zB(CH2)4B(OH}z doublets B = Me3N,BH2(CH2)4BH2' NMe3 Chapter 3 4-16 A = B6HlO B = BsH9 3-1 a, 0; b,+t,-i-; c,f, O,+!, -!; C = B4HlO d,e, 1, -!; f, -i-, +i- 4-17 A = Me1 BH1 BH2 (b) a, -1; b, -!; c, 0, -!; d, B = (EtNBHh _.J. _1. 3' 6 C = trans- (Me2BN :CHMejz (c) -1,0, +! D = cis- (Me1BN :CHMe)z (d) B(l, 3, 6, 9), 0; B(2, 4), -~; B(5, 7, 8, 10), +t Chapter 5 3-3 (201O)B 1H 4 , (1020)B1H;-, 5-3 AHa = 4618kJ/mole (1012)B1HS", (1004)B2Hi, E(AIHB) = 395 kJ/mole (OO30)B2H~ -, (OO22)B2H~ -, 5-4 (a) Me2AIPh1AIMe1 (OO14)B2H~ -, etc. (b) reactants plus 3-4 (1301) and (221O)B4H 6, Me2AIBrClAIMe2 (4004)B4H 11. (1401) and (c)AI(BH4h +Me.B2H6-n (2301)B sH7, and (5013) and (d) AI( °Hh + methane (e,!) (4104)BsH 13 appear possible Me2AIMe(OBu')AIMe1 3-6 (2402) and (3311)B6HlO are 5-7 B, 97; AI, 78; Ga, 34 theoretically possible, as are (3303), (4212), (5121), and Chapter 6 (6030)B 6H 12. -

(12) United States Patent (10) Patent No.: US 9,023,240 B2 Fujinaga Et Al
USOO9023240B2 (12) United States Patent (10) Patent No.: US 9,023,240 B2 Fujinaga et al. (45) Date of Patent: *May 5, 2015 (54) SILICON NITRIDE POWDER FOR 11/7761; C09K11/7774; C09K11/7792; SILICONNITRIDE PHOSPHOR, CAALSIN, C01P 2004/03: C01P 2004/61; C01P 2004/06; PHOSPHORUSING SAME, SRSIN C01P 2006/80; C01P 2002/54; C01P 2004/52; PHOSPHORUSING SAME, (SR, CA)ALSIN, C01P 2006/12: C04B 35/584: CO4B PHOSPHORUSING SAME, LASIN 2235/3878; C04B 2235/5409; C04B PHOSPHORUSING SAME, AND METHODS 223.5/5436; C04B 2235/723; H01L 23/15; FOR PRODUCING THE PHOSPHORS H01L 23/3731; H01L 33/504; C01B 21/0602; C01B 21/068; Y02B 20/181 (75) Inventors: Masataka Fujinaga, Yamaguchi (JP); USPC ....... 252/301.4 F, 301.4 R, 301.6 F; 428/325, Takayuki Ueda, Yamaguchi (JP); 428/402, 698,704 Takuma Sakai, Yamaguchi (JP); See application file for complete search history. Shinsuke Jida, Yamaguchi (JP) (56) References Cited (73) Assignee: UBE Industries, Ltd. (JP) U.S. PATENT DOCUMENTS (*) Notice: Subject to any disclaimer, the term of this patent is extended or adjusted under 35 6,682,663 B2 1/2004 Botty et al. 8, 147,715 B2 * 4/2012 Hirosaki ................. 252/301.4 F U.S.C. 154(b) by 0 days. 2002/0164475 A1* 11/2002 Imamura et al. ... 428/325 2005/0094381 A1* 5/2005 Imamura et al. .............. 361/750 This patent is Subject to a terminal dis 2006/0033083 A1 2/2006 Sakane et al. ........... 252/301.4F claimer. 2009/0134775 A1* 5, 2009 Watanabe et al. ............. 313,503 2009, O250663 A1* 10, 2009 Oshio ................... -

Nanomagnetism in Otherwise Nonmagnetic Materials Tatiana Makarova Umeå University, Umeå, Sweden, and Ioffe Physico-Technical Institute, St
1 Nanomagnetism in Otherwise Nonmagnetic Materials Tatiana Makarova Umeå University, Umeå, Sweden, and Ioffe Physico-Technical Institute, St. Petersburg, Russia. [email protected] This is a 20% excerpt of the manuscript entitled Nanomagnetism in Otherwise Nonmagnetic Materials which has been reviewed and approved for the publication in Handbook of Nanophysics (HNP), 7 Volumes, 300 Chapters Klaus D. Sattler, Editor, Taylor&Francis Publisher (CRC Press). In the present version the Introduction, Tutorial, Figures, Tables, critical analysis and Conclusions are omitted. The manuscript may serve as a source of references. The 20% excerpt is placed on cond-mat ArXiv under the permission of Taylor & Francis Group LLC. Keywords: Dilute magnetic oxides, hexaborides, metal nanoparticles, semiconductor nanoparticles, nanocarbon, graphene, defect-related ferromagnetism. For every complex problem there is an answer that is clear, simple, and wrong. H.L. Mencken 1 What are nonmagnetic materials? 2 Formation of magnetic state through introducing nonmagnetic sp elements to nonmagnetic matrices. 3 Ferromagnetism in hexaborides: discovery – disproof – rebuttal 3.1 Discovery. 3.2 Disproof 3.3 Rebuttal 4 Magnetic semiconducting oxides 4.1 Zink oxide 4.2 Titanium oxide 4.3 Hafnium oxide 4.4 Other nonmagnetic oxides 5 Magnetism in metal nanoparticles 6 Magnetism in semiconductor nanostructures 7 Ferromagnetism in carbon nanostructures 7.1 Pyrolitic carbonaceous materials 7.2 Graphite 7.3 Porous graphite 7.4 Carbon nanoparticles 7.5 Nanographite 7.6 Fullerenes 7.7 Irradiated carbon structures 7.8 Magnetic nature of intrinsic carbon defects 7.9 Magnetism of graphene 8 Possible traps in search of magnetic order 9 Nontrivial role of transition metals. -

Boron-Based Nanomaterials Under Extreme Conditions Remi Grosjean
Boron-based nanomaterials under extreme conditions Remi Grosjean To cite this version: Remi Grosjean. Boron-based nanomaterials under extreme conditions. Chemical Physics [physics.chem-ph]. Université Pierre et Marie Curie - Paris VI, 2016. English. NNT : 2016PA066393. tel-01898865 HAL Id: tel-01898865 https://tel.archives-ouvertes.fr/tel-01898865 Submitted on 19 Oct 2018 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. Université Pierre et Marie Curie Ecole doctorale 397, Chimie et Physico-Chimie des Matériaux Laboratoire Chimie de la Matière Condensée de Paris Institut de Minéralogie, de Physique des Matériaux et de Cosmochimie Nanomatériaux à base de bore sous conditions extrêmes Boron-based nanomaterials under extreme conditions Par Rémi Grosjean Thèse de doctorat en Science des Matériaux Dirigée par David Portehault, Yann Le Godec, Corinne Chanéac Présentée et soutenue publiquement le 17 Octobre 2016 Devant un jury composé de : M. Bernard Samuel Directeur de recherche, CNRS Rapporteur M. Machon Denis Maître de conférences, Université Lyon 1 Rapporteur Mme. Laurencin Danielle Chargée de recherche, CNRS Examinatrice M. Petit Christophe Professeur, Université Paris 6 Examinateur M. Portehault David Chargé de recherche CNRS Co-directeur de thèse M.