VANADIUM PENTOXIDE 1. Exposure Data
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

Vanadium Pentoxide and Other Inorganic Vanadium Compounds
This report contains the collective views of an international group of experts and does not necessarily represent the decisions or the stated policy of the United Nations Environment Programme, the International Labour Organization, or the World Health Organization. Concise International Chemical Assessment Document 29 VANADIUM PENTOXIDE AND OTHER INORGANIC VANADIUM COMPOUNDS Note that the layout and pagination of this pdf file are not identical to the printed CICAD First draft prepared by Dr M. Costigan and Mr R. Cary, Health and Safety Executive, Liverpool, United Kingdom, and Dr S. Dobson, Centre for Ecology and Hydrology, Huntingdon, United Kingdom Published under the joint sponsorship of the United Nations Environment Programme, the International Labour Organization, and the World Health Organization, and produced within the framework of the Inter-Organization Programme for the Sound Management of Chemicals. World Health Organization Geneva, 2001 The International Programme on Chemical Safety (IPCS), established in 1980, is a joint venture of the United Nations Environment Programme (UNEP), the International Labour Organization (ILO), and the World Health Organization (WHO). The overall objectives of the IPCS are to establish the scientific basis for assessment of the risk to human health and the environment from exposure to chemicals, through international peer review processes, as a prerequisite for the promotion of chemical safety, and to provide technical assistance in strengthening national capacities for the sound management -

ANTHRAQUINONE and ANTHRONE SERIES Part IX
ANTHRAQUINONE AND ANTHRONE SERIES Part IX. Chromatographic Adsorption of Aminoanthraquinones on Alumina BY N. R. RAo, K. H. SHAH AND K. VENKATARAMAN, F.A.Sc. (Department of Chemical Technology. University of Bombay, Bombay) Received November 10, 1951 CORRELATIONS between chemical constitution and chromatograpl~c behaviour can only be attempted in molecales of the same or similar types and under specified conditions of chromatographic treatment, since complex solvent- adsorbent, solute-solvent and solute-adsorbent relationships are involved. Changes in the sequence of adsorption of the constituents in a mixture have been observed, ooth when the adsorbent is changed and when the deve- loping solvent is changed. Thus Strain was able tc separate some ternary mixtures in four of the six pessible sequences by changing the solvent1; and LeRosen found that cryptoxanthin is aloove lycopene on alumina and on calcium carbonate columns, but the order is inverted on a column of calcium hydroxide, using benzene as developer with all the three adsoroents. 2 An inversion of the sequence of adsorption of N-ethyl-N, N'-diphenylurea and 4-nitro-N-ethylaniline was observed by Schroeder when a slight alteration in the composition of the developer (2 or 5~o of ethyl acetate in light petro- leum) was made. 3 LeRosen has defined the adsorption affinity R as the rate of movement of the adsorptive zone (mm./min.) divided by the rate of flow of developing solvent. 2 R values have been used for standardizing adsorbents and for determining the efficiency of developers; using the same adsorbent and solvent, R values provide a quantitative measure of the adsorption affinities of a series of compounds which can then be correlated with their chemical constitution. -

Kinetic Studies of Sodium and Metforminium Decavanadates Decomposition and in Vitro Cytotoxicity and Insulin- Like Activity
inorganics Article Kinetic Studies of Sodium and Metforminium Decavanadates Decomposition and In Vitro Cytotoxicity and Insulin- Like Activity Aniela M. Silva-Nolasco 1,2, Luz Camacho 2 , Rafael Omar Saavedra-Díaz 1, Oswaldo Hernández-Abreu 1 , Ignacio E. León 3 and Irma Sánchez-Lombardo 1,* 1 Centro de Investigación de Ciencia y Tecnología Aplicada de Tabasco, División Académica de Ciencias Básicas (CICTAT), Universidad Juárez Autónoma de Tabasco, Carretera Cunduacán-Jalpa km. 1 Col. La Esmeralda, Cunduacán 86690, Tabasco, Mexico; [email protected] (A.M.S.-N.); [email protected] (R.O.S.-D.); [email protected] (O.H.-A.) 2 Laboratorio de Nutrición Experimental, Instituto Nacional de Pediatría, Ciudad de Mexico 04530, Mexico; [email protected] 3 Centro de Química Inorgánica CEQUINOR (CONICET, UNLP), Bv 120 1465, La Plata 1900, Argentina; [email protected] * Correspondence: [email protected] Received: 22 October 2020; Accepted: 2 December 2020; Published: 8 December 2020 Abstract: The kinetics of the decomposition of 0.5 and 1.0 mM sodium decavanadate (NaDeca) and metforminium decavanadate (MetfDeca) solutions were studied by 51V NMR in Dulbecco’s modified Eagle’s medium (DMEM) medium (pH 7.4) at 25 ◦C. The results showed that decomposition products 2 4 are orthovanadate [H2VO4]− (V1) and metavanadate species like [H2V2O7] − (V2), [V4O12] − (V4) 5 and [V5O15] − (V5) for both compounds. The calculated half-life times of the decomposition reaction were 9 and 11 h for NaDeca and MetfDeca, respectively, at 1 mM concentration. The hydrolysis products that presented the highest rate constants were V1 and V4 for both compounds. -
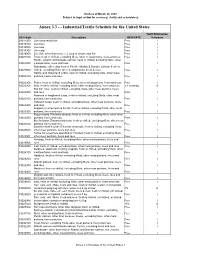
US Schedule for Internet V2
Draft as of March 23, 2007 Subject to legal review for accuracy, clarity and consistency. Annex 3.3 - - Industrial/Textile Schedule for the United States Tariff Elimination US 8 digit Description MFN RATE Schedule 03011000 Live ornamental fish Free I 03019100 Live trout Free I 03019200 Live eels Free I 03019300 Live carp Free I 03019900 Live fish, other than trout, eel, carp or ornamental fish Free I 03021100 Trout, fresh or chilled, excluding fillets, other meat portions, livers and roes Free I Pacific, Atlantic and Danube salmon, fresh or chilled, excluding fillets, other 03021200 meat portions, livers and roes Free I Salmonidae other than trout or Pacific, Atlantic & Danube salmon, fresh or 03021900 chilled, excluding fillets, other meat portions, livers & roes Free I Halibut and Greenland turbot, fresh or chilled, excluding fillets, other meat 03022100 portions, livers and roes Free I 03022200 Plaice, fresh or chilled, excluding fillets, other meat portions, livers and roes Free I 03022300 Sole, fresh or chilled, excluding fillets, other meat portions, livers and roes 1.1 cents/kg A Flat fish, nesi, fresh or chilled, excluding fillets, other meat portions, livers 03022900 and roes Free I Albacore or longfinned tunas, fresh or chilled, excluding fillets, other meat 03023100 portions, livers and roes Free I Yellowfin tunas, fresh or chilled, excluding fillets, other meat portions, livers 03023200 and roes Free I Skipjack or stripe-bellied bonito, fresh or chilled, excluding fillets, other meat 03023300 portions, livers and roes Free -

PROVISIONAL PEER-REVIEWED TOXICITY VALUES for VANADIUM and ITS SOLUBLE INORGANIC COMPOUNDS OTHER THAN VANADIUM PENTOXIDE (CASRN 7440-62-2 and Others)
EPA/690/R-09/070F l Final 9-30-2009 Provisional Peer-Reviewed Toxicity Values for Vanadium and Its Soluble Inorganic Compounds Other Than Vanadium Pentoxide (CASRN 7440-62-2 and Others) Derivation of Subchronic and Chronic Oral RfDs Superfund Health Risk Technical Support Center National Center for Environmental Assessment Office of Research and Development U.S. Environmental Protection Agency Cincinnati, OH 45268 Commonly Used Abbreviations BMD Benchmark Dose IRIS Integrated Risk Information System IUR inhalation unit risk LOAEL lowest-observed-adverse-effect level LOAELADJ LOAEL adjusted to continuous exposure duration LOAELHEC LOAEL adjusted for dosimetric differences across species to a human NOAEL no-observed-adverse-effect level NOAELADJ NOAEL adjusted to continuous exposure duration NOAELHEC NOAEL adjusted for dosimetric differences across species to a human NOEL no-observed-effect level OSF oral slope factor p-IUR provisional inhalation unit risk p-OSF provisional oral slope factor p-RfC provisional inhalation reference concentration p-RfD provisional oral reference dose RfC inhalation reference concentration RfD oral reference dose UF uncertainty factor UFA animal to human uncertainty factor UFC composite uncertainty factor UFD incomplete to complete database uncertainty factor UFH interhuman uncertainty factor UFL LOAEL to NOAEL uncertainty factor UFS subchronic to chronic uncertainty factor i FINAL 9-30-2009 PROVISIONAL PEER-REVIEWED TOXICITY VALUES FOR VANADIUM AND ITS SOLUBLE INORGANIC COMPOUNDS OTHER THAN VANADIUM PENTOXIDE (CASRN 7440-62-2 and others) Background On December 5, 2003, the U.S. Environmental Protection Agency's (U.S. EPA) Office of Superfund Remediation and Technology Innovation (OSRTI) revised its hierarchy of human health toxicity values for Superfund risk assessments, establishing the following three tiers as the new hierarchy: 1) U.S. -

1 Abietic Acid R Abrasive Silica for Polishing DR Acenaphthene M (LC
1 abietic acid R abrasive silica for polishing DR acenaphthene M (LC) acenaphthene quinone R acenaphthylene R acetal (see 1,1-diethoxyethane) acetaldehyde M (FC) acetaldehyde-d (CH3CDO) R acetaldehyde dimethyl acetal CH acetaldoxime R acetamide M (LC) acetamidinium chloride R acetamidoacrylic acid 2- NB acetamidobenzaldehyde p- R acetamidobenzenesulfonyl chloride 4- R acetamidodeoxythioglucopyranose triacetate 2- -2- -1- -β-D- 3,4,6- AB acetamidomethylthiazole 2- -4- PB acetanilide M (LC) acetazolamide R acetdimethylamide see dimethylacetamide, N,N- acethydrazide R acetic acid M (solv) acetic anhydride M (FC) acetmethylamide see methylacetamide, N- acetoacetamide R acetoacetanilide R acetoacetic acid, lithium salt R acetobromoglucose -α-D- NB acetohydroxamic acid R acetoin R acetol (hydroxyacetone) R acetonaphthalide (α)R acetone M (solv) acetone ,A.R. M (solv) acetone-d6 RM acetone cyanohydrin R acetonedicarboxylic acid ,dimethyl ester R acetonedicarboxylic acid -1,3- R acetone dimethyl acetal see dimethoxypropane 2,2- acetonitrile M (solv) acetonitrile-d3 RM acetonylacetone see hexanedione 2,5- acetonylbenzylhydroxycoumarin (3-(α- -4- R acetophenone M (LC) acetophenone oxime R acetophenone trimethylsilyl enol ether see phenyltrimethylsilyl... acetoxyacetone (oxopropyl acetate 2-) R acetoxybenzoic acid 4- DS acetoxynaphthoic acid 6- -2- R 2 acetylacetaldehyde dimethylacetal R acetylacetone (pentanedione -2,4-) M (C) acetylbenzonitrile p- R acetylbiphenyl 4- see phenylacetophenone, p- acetyl bromide M (FC) acetylbromothiophene 2- -5- -

Vanadium in Biosphere and Its Role in Biological Processes
Biological Trace Element Research https://doi.org/10.1007/s12011-018-1289-y Vanadium in Biosphere and Its Role in Biological Processes Deepika Tripathi1 & Veena Mani1 & Ravi Prakash Pal1 Received: 9 July 2017 /Accepted: 26 February 2018 # Springer Science+Business Media, LLC, part of Springer Nature 2018 Abstract Ultra-trace elements or occasionally beneficial elements (OBE) are the new categories of minerals including vanadium (V). The importance of V is attributed due to its multifaceted biological roles, i.e., glucose and lipid metabolism as an insulin-mimetic, antilipemic and a potent stress alleviating agent in diabetes when vanadium is administered at lower doses. It competes with iron for transferrin (binding site for transportation) and with lactoferrin as it is secreted in milk also. The intracellular enzyme protein tyrosine phosphatase, causing the dephosphorylation at beta subunit of the insulin receptor, is inhibited by vanadium, thus facilitating the uptake of glucose inside the cell but only in the presence of insulin. Vanadium could be useful as a potential immune-stimulating agent and also as an antiinflammatory therapeutic metallodrug targeting various diseases. Physiological state and dose of vanadium compounds hold importance in causing toxicity also. Research has been carried out mostly on laboratory animals but evidence for vanadium importance as a therapeutic agent are available in humans and large animals also. This review examines the potential biochemical and molecular role, possible kinetics and distribution, essentiality, immunity, and toxicity- related study of vanadium in a biological system. Keywords Metabolic role . Insulin-mimetic . Biological function . Vanadium Introduction essential elements^ which include aluminum (Al), arsenic (As), cobalt (Co), chromium (Cr), fluorine (F), molybdenum Minerals are inorganic elements, without carbon, found in (Mo), nickel (Ni), silicon (Si), tin (Sn), and vanadium (V) [4]. -

Sports Nutrition: a Review of Selected Nutritional Supplements for Bodybuilders and Strength Athletes
Sports Nutrition: A Review of Selected Nutritional Supplements For Bodybuilders and Strength Athletes Gregory S. Kelly, N.D. Abstract Because there is widespread belief among athletes that special nutritional practices will enhance their achievements in competition, the use of supplements has become common. Accompanying the growth in supplementation by athletes has been a corresponding increase in exaggerated claims or misleading information. This article reviews several supplements currently popular among bodybuilders and other strength athletes in order to clarify which products can be expected to produce results. Included in the discussion are creatine monohydrate, beta-hydroxy beta-methylbutyrate, whey protein, phosphatidylserine, and selected amino acids and minerals. (Alt Med Rev 1997; 2(3):184-201) Introduction The increased focus on fitness and subsequent research in the exercise field has ex- panded the role of nutrition as it relates to sports performance. Because there is widespread belief among athletes that special nutritional practices will enhance their achievements in com- petition, the use of supplements has become common. Although some of the supplements have proven benefits, historically a great deal of the information on products is either misleading or exaggerated. This is perhaps best witnessed in the products marketed to bodybuilders and other athletes concerned with size, strength, and body composition. This article reviews some of the supplements currently promoted in this market in an effort to determine which contribute to maximizing results. Included in the review are creatine monohydrate, beta-hydroxy beta- methylbutyrate (HMB), whey protein, phosphatidylserine, and selected amino acids and minerals. Creatine Monohydrate Creatine monohydrate has become one of the most popular supplements in the history of bodybuilding. -

Dietary Supplements Compendium Volume 1
2015 Dietary Supplements Compendium DSC Volume 1 General Notices and Requirements USP–NF General Chapters USP–NF Dietary Supplement Monographs USP–NF Excipient Monographs FCC General Provisions FCC Monographs FCC Identity Standards FCC Appendices Reagents, Indicators, and Solutions Reference Tables DSC217M_DSCVol1_Title_2015-01_V3.indd 1 2/2/15 12:18 PM 2 Notice and Warning Concerning U.S. Patent or Trademark Rights The inclusion in the USP Dietary Supplements Compendium of a monograph on any dietary supplement in respect to which patent or trademark rights may exist shall not be deemed, and is not intended as, a grant of, or authority to exercise, any right or privilege protected by such patent or trademark. All such rights and privileges are vested in the patent or trademark owner, and no other person may exercise the same without express permission, authority, or license secured from such patent or trademark owner. Concerning Use of the USP Dietary Supplements Compendium Attention is called to the fact that USP Dietary Supplements Compendium text is fully copyrighted. Authors and others wishing to use portions of the text should request permission to do so from the Legal Department of the United States Pharmacopeial Convention. Copyright © 2015 The United States Pharmacopeial Convention ISBN: 978-1-936424-41-2 12601 Twinbrook Parkway, Rockville, MD 20852 All rights reserved. DSC Contents iii Contents USP Dietary Supplements Compendium Volume 1 Volume 2 Members . v. Preface . v Mission and Preface . 1 Dietary Supplements Admission Evaluations . 1. General Notices and Requirements . 9 USP Dietary Supplement Verification Program . .205 USP–NF General Chapters . 25 Dietary Supplements Regulatory USP–NF Dietary Supplement Monographs . -

Toxicological Profile for Vanadium
VANADIUM 107 4. CHEMICAL AND PHYSICAL INFORMATION 4.1 CHEMICAL IDENTITY Vanadium is a naturally occurring element that appears in group 5(B5) of the periodic table (Lide 2008). Vanadium is widely distributed in the earth’s crust at an average concentration of 100 ppm nd (approximately 100 mg/kg), similar to that of zinc and nickel (Byerrum 1991). Vanadium is the 22 most abundant element in the earth’s crust (Baroch 2006). Vanadium is found in about 65 different minerals; carnotite, roscoelite, vanadinite, and patronite are important sources of this metal along with bravoite and davidite (Baroch 2006, Lide 2008). It is also found in phosphate rock and certain ores and is present in some crude oils as organic complexes (Lide 2008). Table 4-1 lists common synonyms and other pertinent identification information for vanadium and representative vanadium compounds. 4.2 PHYSICAL AND CHEMICAL PROPERTIES Vanadium is a gray metal with a body-centered cubic crystal system. It is a member of the first transition series. Because of its high melting point, it is referred to as a refractory metal (Baroch 2006). When highly pure, it is a bright white metal that is soft and ductile. It has good structural strength and a low- fission neutron cross section. Vanadium has good corrosion resistance to alkalis, sulfuric and hydrochloric acid, and salt water; however, the metal oxidizes readily above 660 °C (Lide 2008). The chemistry of vanadium compounds is related to the oxidation state of the vanadium (Woolery 2005). Vanadium has oxidation states of +2, +3, +4, and +5. When heated in air at different temperatures, it oxidizes to a brownish black trioxide, a blue black tetraoxide, or a reddish orange pentoxide. -

Ferro Vanadium Production Business. Ferrovanadium Market Is Expected to Expand at a CAGR of 5.0% Between 2018 and 2028
www.entrepreneurindia.co Introduction Ferro Vanadium is an alloy which is formed by combining iron and vanadium with a vanadium content range of 35%-85%. Ferro Vanadium is a universal hardener, strengthener and anti-corrosive additive for steels like high-strength low-alloy (HSLA) steel, tool steels, as well as other ferrous-based products. Ferro vanadium belongs to the category of ferroalloy. Ferro vanadium is an alloy which is formed by combining iron and vanadium. Ferrovanadium contains 35% to 85% of vanadium depending on applications of the product in end-use industry. Ferro vanadium is an alloy material that is used in manufacturing of steel. It imparts desirable properties such as abrasion resistance, high temperature and hardenability. www.entrepreneurindia.co Ferro vanadium used for manufacturing of steel offers the end product with high stability against alkalis as well as acids such as sulphuric and hydrochloric acids. In addition, products containing ferro vanadium are at reduced risk to be susceptible to corrosion. Ferro vanadium also helps in reducing the overall weight of the material as well as increasing the overall tensile strength of the end product. In addition, it helps in promoting fine grain size and increasing hardenability through precipitation of nitrides and carbides. Ferro vanadium is manufactured using an electric arc furnace in which scrap iron is melted initially and then it is combined with the mixture of aluminum as well as flux such as calcium fluoride and calcium oxide. It is usually supplied in pallet boxes or in shrink wrapped in super bags. www.entrepreneurindia.co Ferro Vanadium is produced from Vanadium Sludge & usually available in the range with V: 50-85%. -

Opinion on the SCCNFP on Disperse Violet 1 Impurified with Disperse Red
SCCNFP/0504/01, final OPINION OF THE SCIENTIFIC COMMITTEE ON COSMETIC PRODUCTS AND NON-FOOD PRODUCTS INTENDED FOR CONSUMERS CONCERNING DISPERSE VIOLET 1 IMPURIFIED WITH DISPERSE RED 15 Colipa n° C64 & C61 adopted by the SCCNFP during the 19th Plenary meeting of 27 February 2002 SCCNFP/0504/01, final Evaluation and opinion on : Disperse Violet 1 impurified with Disperse Red 15 ____________________________________________________________________________________________ 1. Terms of Reference 1.1 Context of the question The adaptation to technical progress of the Annexes to Council Directive 76/768/EEC of 27 July 1976 on the approximation of the laws of the Member States relating to cosmetic products. 1.2 Request to the SCCNFP The SCCNFP is requested to answer the following questions : * Is Disperse Violet 1 impurified with Disperse Red 15 safe for use in cosmetic products? * Does the SCCNFP propose any restrictions or conditions for its use in cosmetic products? 1.3 Statement on the toxicological evaluation The SCCNFP is the scientific advisory body to the European Commission in matters of consumer protection with respect to cosmetics and non-food products intended for consumers. The Commission’s general policy regarding research on animals supports the development of alternative methods to replace or to reduce animal testing when possible. In this context, the SCCNFP has a specific working group on alternatives to animal testing which, in co-operation with other Commission services such as ECVAM (European Centre for Validation of Alternative Methods), evaluates these methods. The extent to which these validated methods are applicable to cosmetic products and its ingredients is a matter of the SCCNFP.