Stereochemistry Fischer Projections, Absolute Configuration and (R)/(S) Notation Dr Alan Spivey; Office: 834 C1; E-Mail: [email protected]; Tel.: 45841
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

II. Stereochemistry 5
B.Sc.(H) Chemistry Semester - II Core Course - III (CC-III) Organic Chemistry - I II. Stereochemistry 5. Physical and Chemical Properties of Stereoisomers Dr. Rajeev Ranjan University Department of Chemistry Dr. Shyama Prasad Mukherjee University, Ranchi 1 Syllabus & Coverage Syllabus II Stereochemistry: Fischer Projection, Newmann and Sawhorse Projection formulae and their interconversions. Geometrical isomerism: cis–trans and syn-anti isomerism, E/Z notations with Cahn Ingold and Prelog (CIP) rules for determining absolute configuration. Optical Isomerism: Optical Activity, Specific Rotation, Chirality/Asymmetry, Enantiomers, Molecules with two or more chiral-centres, Distereoisomers, Meso structures, Racemic mixture. Resolution of Racemic mixtures. Relative and absolute configuration: D/L and R/S designations. Coverage: 1. Types of Isomers : Comparing Structures 2. Optical Activity 3. Racemic Mixtures : Separation of Racemic Mixtures 4. Enantiomeric Excess and Optical Purity 5. Relative and Absolute Configuration 6. Physical and Chemical Properties of Stereoisomers 2 Stereochemistry Types of Isomers Dr. Rajeev Ranjan 3 Stereochemistry Determining the Relationship Between Two Non-Identical Molecules Dr. Rajeev Ranjan 4 Stereochemistry Comparing Structures: Are the structures connected the same? yes no Are they mirror images? Constitutional Isomers yes no Enantiomers Enantiomers Is there a plane of symmetry? All chiral centers will be opposite between them. yes no Meso Diastereomers superimposable Dr. Rajeev Ranjan 5 Stereochemistry Optical Activity: • The chemical and physical properties of two enantiomers are identical except in their interaction with chiral substances. • The physical property that differs is the behavior when subjected to plane-polarized light ( this physical property is often called an optical property). • Plane-polarized (polarized) light is light that has an electric vector that oscillates in a single plane. -

Influence of Chirality on the Electromagnetic Wave Electrical and Computer Engineering
Influence of Chirality on the Electromagnetic Wave Propagation: Unbounded Media And Chirowaveguides Priyá Dilipa Gaunço Dessai Dissertation submitted to obtain the Master Degree in Electrical and Computer Engineering Jury President: Professor José Manuel Bioucas Dias Supervisor: Professor Carlos Manuel dos Reis Paiva Co- Supervisor: Professor António Luís da Silva Topa Member Professor Sérgio de Almeida Matos December 2011 Abstract When a chiral medium interacts with the polarization state of an electromag- netic plane wave and couples selectively with either the left or right circularly polarized component, we call this property the optical activity. Since the beginning of the 19th century, the study of complex materials has intensied, and the chiral and bi-isotropic (BI) media have generated one of the most interesting and challenging subjects in the electromagnetic research groups in terms of theoretical problems and potential applications. This dissertation addresses the theoretical interaction between waves and the chiral media. From the study of chiral structures it is possible to observe the eect of the polarization rotation, the propagation modes and the cuto frequencies. The reection and transmission coecients between a simple isotropic media (SIM) and chiral media are also analyzed, as well as the relation between the Brewster angle and the chiral parameter. The BI planar structures are also analyzed for a closed guide, the parallel-plate chirowaveguide, and for a semi-closed guide, the grounded chiroslab. From these structures -

Locally Enhanced and Tunable Optical Chirality in Helical Metamaterials
hv photonics Article Locally Enhanced and Tunable Optical Chirality in Helical Metamaterials Philipp Gutsche 1,*, Raquel Mäusle 1 and Sven Burger 1,2 1 Zuse Institute Berlin, Takustr. 7, 14195 Berlin, Germany; [email protected] (R.M.); [email protected] (S.B.) 2 JCMwave GmbH, Bolivarallee 22, 14050 Berlin, Germany * Correspondence: [email protected]; Tel.: +49-30-841-85-203 Received: 31 October 2016; Accepted: 18 November 2016; Published: 23 November 2016 Abstract: We report on a numerical study of optical chirality. Intertwined gold helices illuminated with plane waves concentrate right and left circularly polarized electromagnetic field energy to sub-wavelength regions. These spots of enhanced chirality can be smoothly shifted in position and magnitude by varying illumination parameters, allowing for the control of light-matter interactions on a nanometer scale. Keywords: metamaterials; optical chirality; computational nano-optics 1. Introduction Helical metamaterials strongly impact the optical response of incident circularly (CPL) and linearly polarized light. They serve as efficient circular polarizers [1] and are candidates for chiral near-field sources [2]. Both, advancement of fabrication techniques [3–5] and design which employs fundamental physical properties [6] have significantly increased the performance of these complex structures. Most experimental, numerical and theoretical studies focus on the far-field response of helical metamaterials. Nevertheless, near-field interaction of light and chiral matter is expected to be enhanced in chiral near-fields. Recently, the quantity of optical chirality has been introduced quantifying this phenomenon [7]. In the weak-coupling regime, the interplay of electromagnetic fields and chiral molecules, which are not superimposable with their mirror image, is directly proportional to this near-field measure [8]. -
Marking Scheme for the Exam
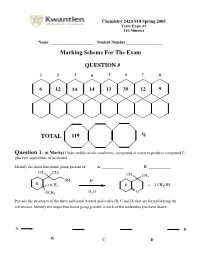
Chemistry 2420 S10 Spring 2005 Term Exam #1 110 Minutes Name: ______________________ Student Number___________________ Marking Scheme For The Exam QUESTION # 1 2 3 4 5 6 7 8 6 12 14 14 13 39 12 9 TOTAL 119 % Question 1. (6 Marks) Under mildly acidic conditions, compound A reacts to produce compound E plus two equivalents of methanol. Identify the main functional group present in: A: ___________ E: ___________ CH3 CH3 CH 3 CH3 + NH2 H A OCH3 E + 2 CH3OH H O OCH3 2 N Provide the structures of the three additional neutral molecules (B, C and D) that are formed during the conversion. Identify the major functional group present in each of the molecules you have drawn. A E B C D Question 2. (12 Marks) Circle the structure that will best satisfy the given information. - the compound able to undergo an intramolecular cyclization to form a hemiacetal H H HO H HO OH O O O - the compound able to undergo an intramolecular cyclization to form an enamine H H H Me2N MeHN H2N O O O + - the produc t formed from treatment of 2-butanone with NaBD4 followed by a H3O work-up DO H HO D DO D HO H - the compound that would most likely be coloured O H O O - the compound capable of undergoing mutarotation O O O OH OCH3 OH CH3 H CH 3 - the compound that would not react with MeMgBr O O O OH OCH3 OH CH3 H CH3 - the compound bes t described as “anti-aromatic”: - the most stable carbocation + + + Cl Cl Cl OCH 3 H NO2 H H H Question 3. -

Chirality in Chemical Molecules
Chirality in Chemical Molecules. Molecules which are active in human physiology largely function as keys in locks. The active molecule is then called a ligand and the lock a receptor. The structures of both are highly specific to the degree that if one atom is positioned in a different position than that required by the receptor to be activated, then no stimulation of the receptor or only partial activation can take place. Again in a similar fashion if one tries to open the front door with a key that looks almost the same than the proper key for that lock, one will usually fail to get inside. A simple aspect like a lengthwise groove on the key which is on the left side instead of the right side, can mean that you can not open the lock if your key is the “chirally incorrect” one. The second aspect to understand is which molecules display chirality and which do not. The word chiral comes from the Greek which means “hand-like”. Our hands are mirror images of each other and as such are not identical. If they were, then we would not need a right hand and left hand glove. We can prove that they are not identical by trying to lay one hand on top of the other palms up. When we attempt to do this, we observe that the thumbs and fingers do not lie on top of one another. We say that they are non-super imposable upon one another. Since they are not the same and yet are mirror images of each other, they are said to exhibit chirality. -

Chapter 4: Stereochemistry Introduction to Stereochemistry
Chapter 4: Stereochemistry Introduction To Stereochemistry Consider two of the compounds we produced while finding all the isomers of C7H16: CH3 CH3 2-methylhexane 3-methylhexane Me Me Me C Me H Bu Bu Me Me 2-methylhexane H H mirror Me rotate Bu Me H 2-methylhexame is superimposable with its mirror image Introduction To Stereochemistry Consider two of the compounds we produced while finding all the isomers of C7H16: CH3 CH3 2-methylhexane 3-methylhexane H C Et Et Me Pr Pr 3-methylhexane Me Me H H mirror Et rotate H Me Pr 2-methylhexame is superimposable with its mirror image Introduction To Stereochemistry Consider two of the compounds we produced while finding all the isomers of C7H16: CH3 CH3 2-methylhexane 3-methylhexane .Compounds that are not superimposable with their mirror image are called chiral (in Greek, chiral means "handed") 3-methylhexane is a chiral molecule. .Compounds that are superimposable with their mirror image are called achiral. 2-methylhexane is an achiral molecule. .An atom (usually carbon) with 4 different substituents is called a stereogenic center or stereocenter. Enantiomers Et Et Pr Pr Me CH3 Me H H 3-methylhexane mirror enantiomers Et Et Pr Pr Me Me Me H H Me H H Two compounds that are non-superimposable mirror images (the two "hands") are called enantiomers. Introduction To Stereochemistry Structural (constitutional) Isomers - Compounds of the same molecular formula with different connectivity (structure, constitution) 2-methylpentane 3-methylpentane Conformational Isomers - Compounds of the same structure that differ in rotation around one or more single bonds Me Me H H H Me H H H H Me H Configurational Isomers or Stereoisomers - Compounds of the same structure that differ in one or more aspects of stereochemistry (how groups are oriented in space - enantiomers or diastereomers) We need a a way to describe the stereochemistry! Me H H Me 3-methylhexane 3-methylhexane The CIP System Revisited 1. -

The Power of Crowding for the Origins of Life
Orig Life Evol Biosph (2014) 44:307–311 DOI 10.1007/s11084-014-9382-5 ORIGIN OF LIFE The Power of Crowding for the Origins of Life Helen Greenwood Hansma Received: 2 October 2014 /Accepted: 2 October 2014 / Published online: 14 January 2015 # Springer Science+Business Media Dordrecht 2015 Abstract Molecular crowding increases the likelihood that life as we know it would emerge. In confined spaces, diffusion distances are shorter, and chemical reactions produce fewer and more regular products. Crowding will occur in the spaces between Muscovite mica sheets, which has many advantages as a site for life’s origins. Keywords Muscovite mica . Molecular crowding . Origin of life . Mechanochemistry. Abiogenesis . Chemical confinement effects . Chirality. Protocells Cells are crowded. Protein molecules in cells are typically so close to each other that there is room for only one protein molecule between them (Phillips, Kondev et al. 2008). This is nothing like a dilute ‘prebiotic soup.’ Therefore, by analogy with living cells, the origins of life were probably also crowded. Molecular Confinement Effects Many chemical reactions are limited by the time needed for reactants to diffuse to each other. Shorter distances speed up these reactions. Molecular complementarity is another principle of life in which pairs or groups of molecules form specific interactions (Root-Bernstein 2012). Current examples are: enzymes & substrates & cofactors; nucleic acid base pairs; antigens & antibodies; nucleic acid - protein interactions. Molecular complementarity is likely to have been involved at life’s origins and also benefits from crowding. Mineral surfaces are a likely place for life’s origins and for formation of polymeric molecules (Orgel 1998). -

REFERENCE ONLY UNIVERSITY of LONDON THESIS This Thesis
REFERENCE ONLY UNIVERSITY OF LONDON THESIS Degree Year Name of Author 0" * COPYRIGHT This is a thesis accepted for a Higher Degree of the University of London. It is an unpublished typescript and the copyright is held by the author. All persons consulting the thesis must read and abide by the Copyright Declaration below. COPYRIGHT DECLARATION I recognise that the copyright of the above-described thesis rests with the author and that no quotation from it or information derived from it may be published without the prior written consent of the author. LOANS Theses may not be lent to individuals, but the Senate House Library may lend a copy to approved libraries within the United Kingdom, for consultation solely on the premises of those libraries. Application should be made to: Inter-Library Loans, Senate House Library, Senate House, Malet Street, London WC1E 7HU. REPRODUCTION University of London theses may not be reproduced without explicit written permission from the Senate House Library. Enquiries should be addressed to the Theses Section of the Library. Regulations concerning reproduction vary according to the date of acceptance of the thesis and are listed below as guidelines. A. Before 1962. Permission granted only upon the prior written consent of the author. (The Senate House Library will provide addresses where possible). B. 1962- 1974. In many cases the author has agreed to permit copying upon completion of a Copyright Declaration. C. 1975 - 1988. Most theses may be copied upon completion of a Copyright Declaration. D. 1989 onwards. Most theses may be copied. This thesis comes within category D. -

Chiral Metamaterials: Simulations and Experiments
HOME | SEARCH | PACS & MSC | JOURNALS | ABOUT | CONTACT US Chiral metamaterials: simulations and experiments This article has been downloaded from IOPscience. Please scroll down to see the full text article. 2009 J. Opt. A: Pure Appl. Opt. 11 114003 (http://iopscience.iop.org/1464-4258/11/11/114003) The Table of Contents and more related content is available Download details: IP Address: 139.91.179.8 The article was downloaded on 10/11/2009 at 12:27 Please note that terms and conditions apply. IOP PUBLISHING JOURNAL OF OPTICS A: PURE AND APPLIED OPTICS J. Opt. A: Pure Appl. Opt. 11 (2009) 114003 (10pp) doi:10.1088/1464-4258/11/11/114003 REVIEW ARTICLE Chiral metamaterials: simulations and experiments Bingnan Wang1, Jiangfeng Zhou1, Thomas Koschny1,2,3, Maria Kafesaki2,3 and Costas M Soukoulis1,2,3 1 Ames Laboratory and Department of Physics and Astronomy, Iowa State University, Ames, IA 50011, USA 2 Institute of Electronic Structure and Laser, FORTH, Heraklion, Crete, 71110, Greece 3 Department of Materials Science and Technology, University of Crete, Heraklion, Crete, 71110, Greece E-mail: [email protected] Received 20 February 2009, accepted for publication 6 May 2009 Published 16 September 2009 Online at stacks.iop.org/JOptA/11/114003 Abstract Electromagnetic metamaterials are composed of periodically arranged artificial structures. They show peculiar properties, such as negative refraction and super-lensing, which are not seen in natural materials. The conventional metamaterials require both negative and negative μ to achieve negative refraction. Chiral metamaterial is a new class of metamaterials offering a simpler route to negative refraction. -

Enantiomeric Resolution and Absolute Configuration of a Chiral Δ-Lactam
molecules Article Enantiomeric Resolution and Absolute Configuration of a Chiral δ-Lactam, Useful Intermediate for the Synthesis of Bioactive Compounds 1, 1, 1 1 Roberta Listro y, Giacomo Rossino y, Serena Della Volpe , Rita Stabile , Massimo Boiocchi 2 , Lorenzo Malavasi 3, Daniela Rossi 1,* and Simona Collina 1 1 Department of Drug Sciences, University of Pavia, via Taramelli 12, 27100 Pavia, Italy; [email protected] (R.L.); [email protected] (G.R.); [email protected] (S.D.V.); [email protected] (R.S.); [email protected] (S.C.) 2 Centro Grandi Strumenti, University of Pavia, via Bassi 21, 27100 Pavia, Italy; [email protected] 3 Department of Chemistry, University of Pavia, via Taramelli 12, 27100 Pavia, Italy; [email protected] * Correspondence: [email protected] These authors contributed equally to this work. y Academic Editor: Józef Drabowicz Received: 2 December 2020; Accepted: 18 December 2020; Published: 19 December 2020 Abstract: During the past several years, the frequency of discovery of new molecular entities based on γ- or δ-lactam scaffolds has increased continuously. Most of them are characterized by the presence of at least one chiral center. Herein, we present the preparation, isolation and the absolute configuration assignment of enantiomeric 2-(4-bromophenyl)-1-isobutyl-6-oxopiperidin-3-carboxylic acid (trans-1). For the preparation of racemic trans-1, the Castagnoli-Cushman reaction was employed. (Semi)-preparative enantioselective HPLC allowed to obtain enantiomerically pure trans-1 whose absolute configuration was assigned by X-ray diffractometry. Compound (+)-(2R,3R)-1 represents a reference compound for the configurational study of structurally related lactams. -

Part 1 in Our Series of Carbohydrate Lectures. in This Section, You Will Learn About Monosaccharide Structure
Welcome to Part 1 in our series of Carbohydrate lectures. In this section, you will learn about monosaccharide structure. The building blocks of larger carbohydrate polymers. 1 First, let’s review why learning about carbohydrates is important. Carbohydrates are used by biological systems as fuels and energy resources. Carbohydrates typically provide quick energy and are one of the primary energy storage forms in animals. Carbohydrates also provide the precursors to other major macromolecules within the body, including the deoxyribose and ribose required for nucleic acid biosynthesis. Carbohydrates can also provide structural support and cushioning/shock absorption, as well as cell‐cell communication, identification, and signaling. 2 Carbohydrates, as their name implies, are water hydrates of carbon, and they all have the same basic core formula (CH2O)n and are always found in the ratio of 1 carbon to 2 hydrogens to 1 oxygen (1:2:1) making them easy to identify from their molecular formula. 3 Carbohydrates can be divided into subcategories based on their complexity. The simplest carbohydrates are the monosaccharides which are the simple sugars required for the biosynthesis of all the other carbohydrate types. Disaccharides consist of two monosaccharides that have been joined together by a covalent bond called the glycosidic bond. Oligosaccharides are polymers that consist of a few monosaccharides covalently linked together, and Polysaccharides are large polymers that contain hundreds to thousands of monosaccharide units all joined together by glycosidic bonds. The remainder of this lecture will focus on monosaccharides 4 Monosaccharides all have alcohol functional groups associated with them. In addition they also have one additional functional group, either an aldehyde or a ketone. -

Lipids, Carbohydrates, Nucleobases & DNA
Chemistry 2050 “Introduction to Organic Chemistry” Fall Semester 2011 Dr. Rainer Glaser Examination #5: The Final “Lipids, Carbohydrates, Nucleobases & DNA.” Monday, December 12, 2011, 10 am – 12 pm. Name: Answer Key Question 1. Lipids and Detergents. 20 Question 2. Glyceraldehyde and Carbohydrates. 20 Question 3. Nucleobases of DNA and RNA. 20 Question 4. Ribose and Deoxyribose and Their Phosphate Esters. 20 Question 5. Single and Double Strands of DNA. 20 Total 100 — 1 — Question 1. Lipids and Detergents. (20 points) (a) Draw the line segment drawing of glyceryl distearoleate. This fat is the _TRI-ester formed between one molecule of the triol __GLYCEROL_, two molecules of the fatty acid stearic acid, H3C−(CH2)16−COOH, and one molecule of the unsaturated fatty acid oleic acid, H3C−(CH2)7−CH=CH−(CH2)7−COOH. In your drawing, attach the oleic acid to one of the primary alcohols of the triol. Clearly indicate whether the alkene in oleic acid is cis or trans. To convert this fat into soap, one would cook the fat with aqueous NaOH solution, a process that is called __SAPONIFICATION__. (12 points) (b) A simple micelle is shown schematically on the right. Each circle signifies a _________ (polar, nonpolar) head-group, and each wiggly line signifies a ________ (polar, nonpolar) alkyl chain. In the case of “normal” soap, the head- group is a ___CARBOXYLATE__ anion. Indicate where we would find the associated cations (i.e., sodium cations; draw a few in the scheme). Water is on the __________ (inside, outside) of this micelle and fats will accumulate on the _________ (inside, outside) of this micelle.