Locally Enhanced and Tunable Optical Chirality in Helical Metamaterials
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Influence of Chirality on the Electromagnetic Wave Electrical and Computer Engineering
Influence of Chirality on the Electromagnetic Wave Propagation: Unbounded Media And Chirowaveguides Priyá Dilipa Gaunço Dessai Dissertation submitted to obtain the Master Degree in Electrical and Computer Engineering Jury President: Professor José Manuel Bioucas Dias Supervisor: Professor Carlos Manuel dos Reis Paiva Co- Supervisor: Professor António Luís da Silva Topa Member Professor Sérgio de Almeida Matos December 2011 Abstract When a chiral medium interacts with the polarization state of an electromag- netic plane wave and couples selectively with either the left or right circularly polarized component, we call this property the optical activity. Since the beginning of the 19th century, the study of complex materials has intensied, and the chiral and bi-isotropic (BI) media have generated one of the most interesting and challenging subjects in the electromagnetic research groups in terms of theoretical problems and potential applications. This dissertation addresses the theoretical interaction between waves and the chiral media. From the study of chiral structures it is possible to observe the eect of the polarization rotation, the propagation modes and the cuto frequencies. The reection and transmission coecients between a simple isotropic media (SIM) and chiral media are also analyzed, as well as the relation between the Brewster angle and the chiral parameter. The BI planar structures are also analyzed for a closed guide, the parallel-plate chirowaveguide, and for a semi-closed guide, the grounded chiroslab. From these structures -

Chirality in Chemical Molecules
Chirality in Chemical Molecules. Molecules which are active in human physiology largely function as keys in locks. The active molecule is then called a ligand and the lock a receptor. The structures of both are highly specific to the degree that if one atom is positioned in a different position than that required by the receptor to be activated, then no stimulation of the receptor or only partial activation can take place. Again in a similar fashion if one tries to open the front door with a key that looks almost the same than the proper key for that lock, one will usually fail to get inside. A simple aspect like a lengthwise groove on the key which is on the left side instead of the right side, can mean that you can not open the lock if your key is the “chirally incorrect” one. The second aspect to understand is which molecules display chirality and which do not. The word chiral comes from the Greek which means “hand-like”. Our hands are mirror images of each other and as such are not identical. If they were, then we would not need a right hand and left hand glove. We can prove that they are not identical by trying to lay one hand on top of the other palms up. When we attempt to do this, we observe that the thumbs and fingers do not lie on top of one another. We say that they are non-super imposable upon one another. Since they are not the same and yet are mirror images of each other, they are said to exhibit chirality. -

Chapter 4: Stereochemistry Introduction to Stereochemistry
Chapter 4: Stereochemistry Introduction To Stereochemistry Consider two of the compounds we produced while finding all the isomers of C7H16: CH3 CH3 2-methylhexane 3-methylhexane Me Me Me C Me H Bu Bu Me Me 2-methylhexane H H mirror Me rotate Bu Me H 2-methylhexame is superimposable with its mirror image Introduction To Stereochemistry Consider two of the compounds we produced while finding all the isomers of C7H16: CH3 CH3 2-methylhexane 3-methylhexane H C Et Et Me Pr Pr 3-methylhexane Me Me H H mirror Et rotate H Me Pr 2-methylhexame is superimposable with its mirror image Introduction To Stereochemistry Consider two of the compounds we produced while finding all the isomers of C7H16: CH3 CH3 2-methylhexane 3-methylhexane .Compounds that are not superimposable with their mirror image are called chiral (in Greek, chiral means "handed") 3-methylhexane is a chiral molecule. .Compounds that are superimposable with their mirror image are called achiral. 2-methylhexane is an achiral molecule. .An atom (usually carbon) with 4 different substituents is called a stereogenic center or stereocenter. Enantiomers Et Et Pr Pr Me CH3 Me H H 3-methylhexane mirror enantiomers Et Et Pr Pr Me Me Me H H Me H H Two compounds that are non-superimposable mirror images (the two "hands") are called enantiomers. Introduction To Stereochemistry Structural (constitutional) Isomers - Compounds of the same molecular formula with different connectivity (structure, constitution) 2-methylpentane 3-methylpentane Conformational Isomers - Compounds of the same structure that differ in rotation around one or more single bonds Me Me H H H Me H H H H Me H Configurational Isomers or Stereoisomers - Compounds of the same structure that differ in one or more aspects of stereochemistry (how groups are oriented in space - enantiomers or diastereomers) We need a a way to describe the stereochemistry! Me H H Me 3-methylhexane 3-methylhexane The CIP System Revisited 1. -

The Power of Crowding for the Origins of Life
Orig Life Evol Biosph (2014) 44:307–311 DOI 10.1007/s11084-014-9382-5 ORIGIN OF LIFE The Power of Crowding for the Origins of Life Helen Greenwood Hansma Received: 2 October 2014 /Accepted: 2 October 2014 / Published online: 14 January 2015 # Springer Science+Business Media Dordrecht 2015 Abstract Molecular crowding increases the likelihood that life as we know it would emerge. In confined spaces, diffusion distances are shorter, and chemical reactions produce fewer and more regular products. Crowding will occur in the spaces between Muscovite mica sheets, which has many advantages as a site for life’s origins. Keywords Muscovite mica . Molecular crowding . Origin of life . Mechanochemistry. Abiogenesis . Chemical confinement effects . Chirality. Protocells Cells are crowded. Protein molecules in cells are typically so close to each other that there is room for only one protein molecule between them (Phillips, Kondev et al. 2008). This is nothing like a dilute ‘prebiotic soup.’ Therefore, by analogy with living cells, the origins of life were probably also crowded. Molecular Confinement Effects Many chemical reactions are limited by the time needed for reactants to diffuse to each other. Shorter distances speed up these reactions. Molecular complementarity is another principle of life in which pairs or groups of molecules form specific interactions (Root-Bernstein 2012). Current examples are: enzymes & substrates & cofactors; nucleic acid base pairs; antigens & antibodies; nucleic acid - protein interactions. Molecular complementarity is likely to have been involved at life’s origins and also benefits from crowding. Mineral surfaces are a likely place for life’s origins and for formation of polymeric molecules (Orgel 1998). -

Chiral Metamaterials: Simulations and Experiments
HOME | SEARCH | PACS & MSC | JOURNALS | ABOUT | CONTACT US Chiral metamaterials: simulations and experiments This article has been downloaded from IOPscience. Please scroll down to see the full text article. 2009 J. Opt. A: Pure Appl. Opt. 11 114003 (http://iopscience.iop.org/1464-4258/11/11/114003) The Table of Contents and more related content is available Download details: IP Address: 139.91.179.8 The article was downloaded on 10/11/2009 at 12:27 Please note that terms and conditions apply. IOP PUBLISHING JOURNAL OF OPTICS A: PURE AND APPLIED OPTICS J. Opt. A: Pure Appl. Opt. 11 (2009) 114003 (10pp) doi:10.1088/1464-4258/11/11/114003 REVIEW ARTICLE Chiral metamaterials: simulations and experiments Bingnan Wang1, Jiangfeng Zhou1, Thomas Koschny1,2,3, Maria Kafesaki2,3 and Costas M Soukoulis1,2,3 1 Ames Laboratory and Department of Physics and Astronomy, Iowa State University, Ames, IA 50011, USA 2 Institute of Electronic Structure and Laser, FORTH, Heraklion, Crete, 71110, Greece 3 Department of Materials Science and Technology, University of Crete, Heraklion, Crete, 71110, Greece E-mail: [email protected] Received 20 February 2009, accepted for publication 6 May 2009 Published 16 September 2009 Online at stacks.iop.org/JOptA/11/114003 Abstract Electromagnetic metamaterials are composed of periodically arranged artificial structures. They show peculiar properties, such as negative refraction and super-lensing, which are not seen in natural materials. The conventional metamaterials require both negative and negative μ to achieve negative refraction. Chiral metamaterial is a new class of metamaterials offering a simpler route to negative refraction. -

Metamaterials with Magnetism and Chirality
1 Topical Review 2 Metamaterials with magnetism and chirality 1 2;3 4 3 Satoshi Tomita , Hiroyuki Kurosawa Tetsuya Ueda , Kei 5 4 Sawada 1 5 Graduate School of Materials Science, Nara Institute of Science and Technology, 6 8916-5 Takayama, Ikoma, Nara 630-0192, Japan 2 7 National Institute for Materials Science, 1-1 Namiki, Tsukuba, Ibaraki 305-0044, 8 Japan 3 9 Advanced ICT Research Institute, National Institute of Information and 10 Communications Technology, Kobe, Hyogo 651-2492, Japan 4 11 Department of Electrical Engineering and Electronics, Kyoto Institute of 12 Technology, Matsugasaki, Sakyo, Kyoto 606-8585, Japan 5 13 RIKEN SPring-8 Center, 1-1-1 Kouto, Sayo, Hyogo 679-5148, Japan 14 E-mail: [email protected] 15 November 2017 16 Abstract. This review introduces and overviews electromagnetism in structured 17 metamaterials with simultaneous time-reversal and space-inversion symmetry breaking 18 by magnetism and chirality. Direct experimental observation of optical magnetochiral 19 effects by a single metamolecule with magnetism and chirality is demonstrated 20 at microwave frequencies. Numerical simulations based on a finite element 21 method reproduce well the experimental results and predict the emergence of giant 22 magnetochiral effects by combining resonances in the metamolecule. Toward the 23 magnetochiral effects at higher frequencies than microwaves, a metamolecule is 24 miniaturized in the presence of ferromagnetic resonance in a cavity and coplanar 25 waveguide. This work opens the door to the realization of a one-way mirror and 26 synthetic gauge fields for electromagnetic waves. 27 Keywords: metamaterials, symmetry breaking, magnetism, chirality, magneto-optical 28 effects, optical activity, magnetochiral effects, synthetic gauge fields 29 Submitted to: J. -

Supramolecular Chirality of Hydrogen-Bonded Rosette Assemblies Mercedes Crego-Calama
Supramolecular chirality of hydrogen-bonded rosette assemblies Mercedes Crego-Calama To cite this version: Mercedes Crego-Calama. Supramolecular chirality of hydrogen-bonded rosette assemblies. Supramolecular Chemistry, Taylor & Francis: STM, Behavioural Science and Public Health Titles, 2007, 19 (01-02), pp.95-106. 10.1080/10610270600981716. hal-00513491 HAL Id: hal-00513491 https://hal.archives-ouvertes.fr/hal-00513491 Submitted on 1 Sep 2010 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. Supramolecular Chemistry For Peer Review Only Supramolecular chirality of hydrogen-bonded rosette assemblies Journal: Supramolecular Chemistry Manuscript ID: GSCH-2006-0028 Manuscript Type: Review Date Submitted by the 31-May-2006 Author: Complete List of Authors: crego-calama, mercedes; University of Twente, SMCT Supramolecular chirality, noncovalent synthesis, amplification of Keywords: chirality, diastereomeric synthesis, enantiomeric synthesis Note: The following files were submitted by the author for peer review, but cannot be converted to PDF. You must view these files (e.g. movies) online. tif.sit URL: http:/mc.manuscriptcentral.com/tandf/gsch Email: [email protected] Page 1 of 20 Supramolecular Chemistry 1 2 3 4 5 Supramolecular chirality of hydrogen-bonded 6 7 rosette assemblies 8 9 10 Socorro Vázquez-Campos, Mercedes Crego-Calama*, and David N. -

Chirality: the Handedness of Molecules
06 Chirality: The Handedness of Molecules Tartaric acid is found in grapes and other fruits, both free and as its salts (see Section 6.4B). Inset: A model of tartaric acid. (© fatihhoca/iStockphoto) KEY QUESTIONS 6.1 What Are Stereoisomers? 6.9 What Is the Significance of Chirality in the 6.2 What Are Enantiomers? Biological World? 6.3 How Do We Designate the Configuration of a 6.10 How Can Enantiomers Be Resolved? Stereocenter? HOW TO 6.4 What Is the 2n Rule? 6.1 How to Draw Enantiomers 6.5 How Do We Describe the Chirality of 6.2 How to Determine the R & S Configuration Cyclic Molecules with Two Stereocenters? without Rotating the Molecule 6.6 How Do We Describe the Chirality 6.3 How to Determine Whether Two Compounds Are of Molecules with Three or More the Same, Enantiomers, or Diastereomers without Stereocenters? the Need to Spatially Manipulate the Molecule 6.7 What Are the Properties of Stereoisomers? 6.8 How Is Chirality Detected in the CHEMICAL CONNECTIONS Laboratory? 6A Chiral Drugs IN THIS CHAPTER, we will explore the relationships between three-dimensional ob- jects and their mirror images. When you look in a mirror, you see a reflection, or mirror Mirror image The reflection image, of yourself. Now, suppose your mirror image becomes a three-dimensional object. of an object in a mirror. 167 168 CHAPTER 6 Chirality: The Handedness of Molecules We could then ask, “What is the relationship between you and your mirror image?” By relationship, we mean “Can your reflection be superposed on the original ‘you’ in such a way that every detail of the reflection corresponds exactly to the original?” The answer is that you and your mirror image are not superposable. -

The Origin of Biological Homochirality
Downloaded from http://cshperspectives.cshlp.org/ on October 4, 2021 - Published by Cold Spring Harbor Laboratory Press The Origin of Biological Homochirality Donna G. Blackmond Department of Chemistry, The Scripps Research Institute, La Jolla, California 92037 Correspondence: [email protected] SUMMARY The fact that sugars, amino acids, and the biological polymers they construct exist exclusively in one of two possible mirror-image forms has fascinated scientists and laymen alike for more than a century. Yet, it was only in the late 20th century that experimental studies began to probe how biological homochirality, a signature of life, arose from a prebiotic world that presumably contained equal amounts of both mirror-image forms of these molecules. This review discusses experimental studies aimed at understanding how chemical reactions, physical processes, or a combination of both may provide prebiotically relevant mechanisms for the enrichment of one form of a chiral molecule over the other to allow for the emergence of biological homochirality. Outline 1 Introduction 5 Combining physical and chemical approaches 2 Models for the origin of homochirality 6 Concluding remarks 3 Chemical models References 4 Physical models Editors: Thomas R. Cech, Joan A. Steitz, and John F. Atkins Additional Perspectives on RNA Worlds available at www.cshperspectives.org Copyright # 2019 Cold Spring Harbor Laboratory Press; all rights reserved; doi: 10.1101/cshperspect.a032540 Cite this article as Cold Spring Harb Perspect Biol 2019;11:a032540 1 Downloaded from http://cshperspectives.cshlp.org/ on October 4, 2021 - Published by Cold Spring Harbor Laboratory Press D.G. Blackmond 1 INTRODUCTION (Roozeboom 1899) a half century before the macroscopic helicity of biopolymers was characterized (Pauling et al. -

Chapter 5: Stereoisomerism- Three-Dimensional Arrangement of Atoms (Groups) in Space 5.1 Overview of Isomerism
Chapter 5: Stereoisomerism- three-dimensional arrangement of atoms (groups) in space 5.1 Overview of Isomerism Isomers: different chemical compounds with the same formula Constitutional isomers: same formula, but different connectivity of atoms (or groups) C5H12 C4H10O C4H11N NH O 2 OH NH2 butanol diethyl ether different carbon skeleton different functional group different position of FG Stereoisomers: molecules with the same connectivity but different spatial arrangement of atoms (groups) H C CH 3 3 H3C H H H H CH3 cis-1,2-dimethylcyclopropane trans-1,2-dimethylcyclopropane H H H CH3 H C CH H C H 3 3 3 93 cis-2-butene trans-2-butene 5.2 Introduction to Stereoisomerism Enantiomers: non-superimposable mirror image isomers. Enantiomers are related to each other much like a right hand is related to a left hand Enantiomers have identical physical properties, e.g., bp, mp, etc. Chirality (derived from the Greek word for hand). Enantiomers are said to be chiral. 94 47 Chirality Center: Chiral molecules most commonly contain a carbon with four different groups; the carbon is referred to as a chiral center, or asymmetric center, or stereogenic center, or stereocenter. H H HO OH CO2H HO2C H3C CH3 H H H CO2H HO HO CO2H CO2H H3C H3C H3C HO CO2H H HO H3C H H H OH HO HO CO2H CO2H H3C H3C HO H CH3 CO2H HO2C H3C 95 5.3 Designating Configuration Using the Cahn-Ingold-Prelog System – Assigning the Absolute Configuration 1a. Look at the four atoms directly attached to the chiral carbon atom and rank them according to decreasing atomic number. -

7.3 Designating Configuration of Enantiomers
Hornback_Ch07_219-256 12/14/04 7:00 PM Page 224 224 CHAPTER 7 I STEREOCHEMISTRY II PROBLEM 7.3 Indicate whether each of these objects or molecules has a plane of symmetry: a) Idealized human face b) Pencil c) Ear Cl CH3 O d) e) f) H3CCH3 O Cl± Br± CH g) C 3 h) C CH3 ± H H Cl Cl ± 7.3 Designating Configuration of Enantiomers Suppose we are working with one enantiomer of a chiral compound. How can we indi- cate which enantiomer is being used when writing about it? Or how can we look up the properties of this enantiomer in a reference book? We need a method to designate the configuration of the enantiomer, to denote the three-dimensional arrangement of the four groups around the chirality center, other than drawing the structure. In the case of many everyday chiral objects, the terms right and left are used, as in a left shoe or right- handed golf clubs. In Section 6.2 we learned how to designate the configuration of geo- metrical isomers using the Cahn-Ingold-Prelog sequence rules and the labels Z and E. The method to designate the configuration of enantiomers uses these same rules. The following steps are used to assign the configuration of a chiral compound: STEP 1 Assign priorities from 1 through 4 to the four groups bonded to the chirality center using the Cahn-Ingold-Prelog sequence rules presented in Section 6.2.The group with the highest priority receives number 1, and the lowest-priority group receives number 4. -
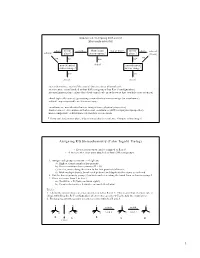
Cahn–Ingold–Prelog)
Handout #6, 5.12 Spring 2003, 2/28/03 Stereochemistry yes mirror zero How many two or more mirror yes achiral achiral plane? stereocenters? plane? (meso) no no no one no* non-identical chiral non-identical mirror image? mirror image? yes yes chiral chiral stereochemistry: study of the spatial characteristics of a molecule stereocenter: atom bonded to four different groups (has R or S configuration) internal mirror plane: plane that divides molecule in such a way that two halves are identical chiral (optically active): possessing a non-identical mirror image (an enantiomer) achiral: superimposable on its mirror image enantiomers: non-identical mirror images (same physical properties) diastereomers: stereoisomers that are not enantiomers (different physical properties) meso compound: achiral molecule that has stereocenters * If you can't find a mirror plane, it doesn't mean that there isn't one. Compare mirror images! Assigning R/S Stereochemistry (Cahn–Ingold–Prelog) • Every stereocenter can be assigned as R or S. • A stereocenter is an atom attached to four different groups. 1. Assign each group a priority (1 = highest). a) Highest atomic number has priority. b) Heavier isotopes have priority (D > H). c) In a tie, move along the chain to the first point of difference. d) With multiple bonds, break each pi-bond and duplicate the atoms at each end. 2. Put the lowest priority group (4) in back and view along the bond from carbon to group 4. 3. Draw an arrow from 1 to 2 to 3. a) Clockwise = R (Your car turns right!) b) Counterclockwise = S (sinister means left in Latin) Tricks: 1.