Comparative Morphometric Study of the Australopithecine Vertebral
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

Dental Pathology, Wear and Developmental Defects in South African Hominins
Dental pathology, wear and developmental defects in South African hominins IAN EDWARD TOWLE A thesis submitted in partial fulfilment of the requirements of Liverpool John Moores University for the degree of Doctor of Philosophy June 2017 Abstract Studying different types of dental pathology, wear, and developmental defects can allow inferences into diet and behaviour in a variety of ways. In this project data on these different variables were collected for South African hominins and compared with extant primates. The species studied include Paranthropus robustus, Australopithecus africanus, A. sediba, early Homo, Homo naledi, baboons, chimpanzees and gorillas. Macroscopic examination of each specimen was performed, with a 10X hand lens used to verify certain pathologies. Variables recorded include antemortem chipping, enamel hypoplasia, caries, occlusal wear, tertiary dentine, abscesses, and periodontal disease. Clear differences in frequencies were found in the different South African hominin species. Homo naledi displays high rates of chipping, especially small fractures above molar wear facets, likely reflecting a diet containing high levels of contaminants. Other noteworthy results include the high levels of pitting enamel hypoplasia in P. robustus molars compared to other species, likely due to a species-specific enamel formation property or developmental disturbance. The low rates of chipping in P. robustus does not fit with this species being a hard food specialist. Instead, the wear best supports a diet of low-quality tough vegetation. Australopithecus africanus likely had a broad diet, with angled molar wear, lack of caries, and high chipping frequencies supporting this conclusion. Seven new carious lesions are described, two from H. naledi and five P. -

The Partial Skeleton Stw 431 from Sterkfontein – Is It Time to Rethink the Plio-Pleistocene Hominin Diversity in South Africa?
doie-pub 10.4436/JASS.98020 ahead of print JASs Reports doi: 10.4436/jass.89003 Journal of Anthropological Sciences Vol. 98 (2020), pp. 73-88 The partial skeleton StW 431 from Sterkfontein – Is it time to rethink the Plio-Pleistocene hominin diversity in South Africa? Gabriele A. Macho1, Cinzia Fornai 2, Christine Tardieu3, Philip Hopley4, Martin Haeusler5 & Michel Toussaint6 1) Earth and Planetary Science, Birkbeck, University of London, London WC1E 7HX, England; School of Archaeology, University of Oxford, Oxford OX1 3QY, England email: [email protected]; [email protected] 2) Institute of Evolutionary Medicine, University of Zurich, Winterthurerstrasse 190, CH-8057 Zurich, Switzerland; Department of Anthropology, University of Vienna, Althanstraße 14, 1090 Vienna, Austria 3) Muséum National d’Histoire Naturelle, 55 rue Buffon, 75005 Paris, France 4) Earth and Planetary Science, Birkbeck, University of London, London WC1E 7HX; Department of Earth Sciences, University College London, London, WC1E 6BT, England 5) Institute of Evolutionary Medicine, University of Zurich, Winterthurerstrasse 190, CH-8057 Zurich, Switzerland 6) retired palaeoanthropologist, Belgium email: [email protected] Summary - The discovery of the nearly complete Plio-Pleistocene skeleton StW 573 Australopithecus prometheus from Sterkfontein Member 2, South Africa, has intensified debates as to whether Sterkfontein Member 4 contains a hominin species other than Australopithecus africanus. For example, it has recently been suggested that the partial skeleton StW 431 should be removed from the A. africanus hypodigm and be placed into A. prometheus. Here we re-evaluate this latter proposition, using published information and new comparative data. Although both StW 573 and StW 431 are apparently comparable in their arboreal (i.e., climbing) and bipedal adaptations, they also show significant morphological differences. -
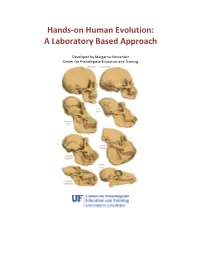
Hands-On Human Evolution: a Laboratory Based Approach
Hands-on Human Evolution: A Laboratory Based Approach Developed by Margarita Hernandez Center for Precollegiate Education and Training Author: Margarita Hernandez Curriculum Team: Julie Bokor, Sven Engling A huge thank you to….. Contents: 4. Author’s note 5. Introduction 6. Tips about the curriculum 8. Lesson Summaries 9. Lesson Sequencing Guide 10. Vocabulary 11. Next Generation Sunshine State Standards- Science 12. Background information 13. Lessons 122. Resources 123. Content Assessment 129. Content Area Expert Evaluation 131. Teacher Feedback Form 134. Student Feedback Form Lesson 1: Hominid Evolution Lab 19. Lesson 1 . Student Lab Pages . Student Lab Key . Human Evolution Phylogeny . Lab Station Numbers . Skeletal Pictures Lesson 2: Chromosomal Comparison Lab 48. Lesson 2 . Student Activity Pages . Student Lab Key Lesson 3: Naledi Jigsaw 77. Lesson 3 Author’s note Introduction Page The validity and importance of the theory of biological evolution runs strong throughout the topic of biology. Evolution serves as a foundation to many biological concepts by tying together the different tenants of biology, like ecology, anatomy, genetics, zoology, and taxonomy. It is for this reason that evolution plays a prominent role in the state and national standards and deserves thorough coverage in a classroom. A prime example of evolution can be seen in our own ancestral history, and this unit provides students with an excellent opportunity to consider the multiple lines of evidence that support hominid evolution. By allowing students the chance to uncover the supporting evidence for evolution themselves, they discover the ways the theory of evolution is supported by multiple sources. It is our hope that the opportunity to handle our ancestors’ bone casts and examine real molecular data, in an inquiry based environment, will pique the interest of students, ultimately leading them to conclude that the evidence they have gathered thoroughly supports the theory of evolution. -

Human Evolution: a Paleoanthropological Perspective - F.H
PHYSICAL (BIOLOGICAL) ANTHROPOLOGY - Human Evolution: A Paleoanthropological Perspective - F.H. Smith HUMAN EVOLUTION: A PALEOANTHROPOLOGICAL PERSPECTIVE F.H. Smith Department of Anthropology, Loyola University Chicago, USA Keywords: Human evolution, Miocene apes, Sahelanthropus, australopithecines, Australopithecus afarensis, cladogenesis, robust australopithecines, early Homo, Homo erectus, Homo heidelbergensis, Australopithecus africanus/Australopithecus garhi, mitochondrial DNA, homology, Neandertals, modern human origins, African Transitional Group. Contents 1. Introduction 2. Reconstructing Biological History: The Relationship of Humans and Apes 3. The Human Fossil Record: Basal Hominins 4. The Earliest Definite Hominins: The Australopithecines 5. Early Australopithecines as Primitive Humans 6. The Australopithecine Radiation 7. Origin and Evolution of the Genus Homo 8. Explaining Early Hominin Evolution: Controversy and the Documentation- Explanation Controversy 9. Early Homo erectus in East Africa and the Initial Radiation of Homo 10. After Homo erectus: The Middle Range of the Evolution of the Genus Homo 11. Neandertals and Late Archaics from Africa and Asia: The Hominin World before Modernity 12. The Origin of Modern Humans 13. Closing Perspective Glossary Bibliography Biographical Sketch Summary UNESCO – EOLSS The basic course of human biological history is well represented by the existing fossil record, although there is considerable debate on the details of that history. This review details both what is firmly understood (first echelon issues) and what is contentious concerning humanSAMPLE evolution. Most of the coCHAPTERSntention actually concerns the details (second echelon issues) of human evolution rather than the fundamental issues. For example, both anatomical and molecular evidence on living (extant) hominoids (apes and humans) suggests the close relationship of African great apes and humans (hominins). That relationship is demonstrated by the existing hominoid fossil record, including that of early hominins. -

Paranthropus Boisei: Fifty Years of Evidence and Analysis Bernard A
Marshall University Marshall Digital Scholar Biological Sciences Faculty Research Biological Sciences Fall 11-28-2007 Paranthropus boisei: Fifty Years of Evidence and Analysis Bernard A. Wood George Washington University Paul J. Constantino Biological Sciences, [email protected] Follow this and additional works at: http://mds.marshall.edu/bio_sciences_faculty Part of the Biological and Physical Anthropology Commons Recommended Citation Wood B and Constantino P. Paranthropus boisei: Fifty years of evidence and analysis. Yearbook of Physical Anthropology 50:106-132. This Article is brought to you for free and open access by the Biological Sciences at Marshall Digital Scholar. It has been accepted for inclusion in Biological Sciences Faculty Research by an authorized administrator of Marshall Digital Scholar. For more information, please contact [email protected], [email protected]. YEARBOOK OF PHYSICAL ANTHROPOLOGY 50:106–132 (2007) Paranthropus boisei: Fifty Years of Evidence and Analysis Bernard Wood* and Paul Constantino Center for the Advanced Study of Hominid Paleobiology, George Washington University, Washington, DC 20052 KEY WORDS Paranthropus; boisei; aethiopicus; human evolution; Africa ABSTRACT Paranthropus boisei is a hominin taxon ers can trace the evolution of metric and nonmetric var- with a distinctive cranial and dental morphology. Its iables across hundreds of thousands of years. This pa- hypodigm has been recovered from sites with good per is a detailed1 review of half a century’s worth of fos- stratigraphic and chronological control, and for some sil evidence and analysis of P. boi se i and traces how morphological regions, such as the mandible and the both its evolutionary history and our understanding of mandibular dentition, the samples are not only rela- its evolutionary history have evolved during the past tively well dated, but they are, by paleontological 50 years. -

Darwin and the Recent African Origin of Modern Humans
EDITORIAL Darwin and the recent African origin of modern humans Richard G. Klein1 Program in Human Biology, Stanford University, Stanford, CA 94305 n this 200th anniversary of When Darwin and Huxley were ac- The Course of Human Evolution Charles Darwin’s birth and tive, many respected scientists sub- In the absence of fossils, Darwin could the 150th anniversary of the scribed to the now discredited idea that not have predicted the fundamental pat- publication of his monumen- human races represented variably tern of human evolution, but his evolu- Otal The Origin of Species (1859) (1), it evolved populations of Homo sapiens. tionary theory readily accommodates seems fitting to summarize Darwin’s The original Neanderthal skull had a the pattern we now recognize. Probably views on human evolution and to show conspicuous browridge, and compared the most fundamental finding is that the how far we have come since. Darwin with the skulls of modern humans, it australopithecines, who existed from at famously neglected the subject in The was decidedly long and low. At the same least 4.5 million to 2 million years ago, Origin, except near the end where he time, it had a large braincase, and Hux- were distinguished from apes primarily noted only that ‘‘light would be thrown ley regarded it as ‘‘the extreme term of by anatomical specializations for habit- on the origin of man and his history’’ by a series leading gradually from it to the ual bipedalism, and it was only after 2 the massive evidence he had compiled highest and best developed of [modern] million years ago that people began to for evolution by means of natural selec- human crania.’’ It was only in 1891 that acquire the other traits, including our tion. -

Virtual Reconstruction of the Australopithecus Africanus Pelvis Sts 65 with Implications for Obstetrics and Locomotion
Journal of Human Evolution 99 (2016) 10e24 Contents lists available at ScienceDirect Journal of Human Evolution journal homepage: www.elsevier.com/locate/jhevol Virtual reconstruction of the Australopithecus africanus pelvis Sts 65 with implications for obstetrics and locomotion * Alexander G. Claxton a, , Ashley S. Hammond b, c, Julia Romano a, Ekaterina Oleinik d, Jeremy M. DeSilva a, e a Department of Anthropology, Boston University, Boston, MA 02215, USA b Center for Advanced Study of Human Paleobiology, Department of Anthropology, The George Washington University, Washington, DC 20052, USA c Department of Anatomy, Howard University College of Medicine, Washington, DC 20059, USA d Scientific Computing and Visualization, Boston University, Boston, MA 02215, USA e Department of Anthropology, Dartmouth College, Hanover, NH 03755, USA article info abstract Article history: Characterizing australopith pelvic morphology has been difficult in part because of limited fossilized Received 24 February 2014 pelvic material. Here, we reassess the morphology of an under-studied adult right ilium and pubis (Sts Accepted 3 June 2016 65) from Member 4 of Sterkfontein, South Africa, and provide a hypothetical digital reconstruction of its overall pelvic morphology. The small size of the pelvis, presence of a preauricular sulcus, and shape of the sciatic notch allow us to agree with past interpretations that Sts 65 likely belonged to a female. The Keywords: morphology of the iliac pillar, while not as substantial as in Homo, is more robust than in A.L. 288-1 and Australopithecus africanus Sts 14. We created a reconstruction of the pelvis by digitally articulating the Sts 65 right ilium and a Sterkfontein Pelvis mirrored copy of the left ilium with the Sts 14 sacrum in Autodesk Maya. -

Pelvic Joint Scaling Relationships and Sacral Shape in Hominoid Primates
Pelvic joint scaling relationships and sacral shape in hominoid primates Ingrid Lundeen Winter 2015 Ingrid Lundeen Introduction Understanding relationships between joints allows inferences to be made about the relative importance of that joint in locomotion. For example, through evolutionary time, there is an overall increase in size of the hind limb joints relative to forelimb joints of bipedal hominins (Jungers, 1988, 1991). These greater hindlimb joint sizes are thought to reflect the higher loading they must bear as posture gradually shifts to rely more on hindlimbs in propulsion, as well as increases in body size through hominin evolution. The first sacral body cross-sectional area in hominins is considered to have expanded over time in response to the higher forces inferred to have been applied by frequent bipedalality and larger body size (Abitbol, 1987; Jungers, 1988; Sanders, 1995; Ruff, 2010). Similarly, the femoral head and acetabular height have increased in size in response to an increase in body during hominin evolution (Ruff, 1988; Jungers, 1991). However, the sacroiliac joint, the intermediate joint between these two force transmission sites, has been less frequently discussed in this evolutionary context (Sanders, 1995). The sacroiliac joint (SIJ) is a synovial, C-shaped joint where the lateral edge of the sacrum and medial edge of the ilium meet. The surface of the SIJ is lined with thick hyaline cartilage on the sacral surface and thin fibrocartilage on the iliac surface (Willard, 2007). The joint is surrounded on all sides by a capsule of strong ligaments bracing the bones against applied forces. At birth, the surface of the SIJ is 2 Ingrid Lundeen flat and smooth but changes after puberty to form slight bumps and grooves that characterize the adult SIJ (Bowen and Cassidy, 1981). -

The Evolution of Humanity
THE EVOLUTION OF HUMANITY: PAST, PRESENT, AND POSSIBLE FUTURE A Review of Humanity’s Taxonomic Classification and Proposal to Classify Humanity as a Sixth Kingdom, Symbolia January 200l John Allen, FLS Global Ecotechnics Corporation 1 Bluebird Court Santa Fe, NM 87508 Email: [email protected] A shorter version of this paper was first published as a philosophic essay in The Duversity Newsletter No.4 (2000) edited and published by the British thinker, AGE Blake. I am taking the step of publishing this expanded paper electronically with fuller scientific details and complete bibliography for the use of any scientist or other thinker or artist or citizen who finds it interesting and will make the proper acknowledgments if they use any part of the paper. I have reviewed the thrust of this full paper with several outstanding scientific thinkers and have been stimulated by their critical feedback. I especially acknowledge stimulating conversations with John Marsden, Sir Ghillean Prance, Tyler Volk, Niles Eldredge, and Abigail Alling, who of course bear no responsibility for any mistakes or any conclusions contained in the paper. Abstract The taxonomy of humans in the teeming world of life forms, has from the beginning of the Theory of Evolution presented one of the most difficult of problems for science. Darwin and Wallace themselves split over this. Darwin opted for a species of primate and Wallace for a difference amounting to a species of a new kingdom. However, a proper taxonomy was probably impossible in their time because the sciences of palaeontology, neurology, ecology, ethnology and archaeology were not available; Darwin was restricted to a choice between dogmatic Biblical and mechanistic world-views. -

A Description of the Geological Context, Discrete Traits, and Linear Morphometrics of the Middle Pleistocene Hominin from Dali, Shaanxi Province, China
AMERICAN JOURNAL OF PHYSICAL ANTHROPOLOGY 150:141–157 (2013) A Description of the Geological Context, Discrete Traits, and Linear Morphometrics of the Middle Pleistocene Hominin from Dali, Shaanxi Province, China Xinzhi Wu1 and Sheela Athreya2* 1Laboratory for Human Evolution, Institute of Vertebrate Paleontology and Paleoanthropology, Chinese Academy of Sciences, Beijing 100044, China 2Department of Anthropology, Texas A&M University, College Station, TX 77843 KEY WORDS Homo heidelbergensis; Homo erectus; Asia ABSTRACT In 1978, a nearly complete hominin Afro/European Middle Pleistocene Homo and align it fossil cranium was recovered from loess deposits at the with Asian H. erectus.Atthesametime,itdisplaysa site of Dali in Shaanxi Province, northwestern China. more derived morphology of the supraorbital torus and It was subsequently briefly described in both English supratoral sulcus and a thinner tympanic plate than and Chinese publications. Here we present a compre- H. erectus, a relatively long upper (lambda-inion) occi- hensive univariate and nonmetric description of the pital plane with a clear separation of inion and opis- specimen and provide comparisons with key Middle thocranion, and an absolute and relative increase in Pleistocene Homo erectus and non-erectus hominins brain size, all of which align it with African and Euro- from Eurasia and Africa. In both respects we find pean Middle Pleistocene Homo. Finally, traits such as affinities with Chinese H. erectus as well as African the form of the frontal keel and the relatively short, and European Middle Pleistocene hominins typically broad midface align Dali specifically with other referred to as Homo heidelbergensis.Specifically,the Chinese specimens from the Middle Pleistocene Dali specimen possesses a low cranial height, relatively and Late Pleistocene, including H. -

Verhaegen M. the Aquatic Ape Evolves
HUMAN EVOLUTION Vol. 28 n.3-4 (237-266) - 2013 Verhaegen M. The Aquatic Ape Evolves: Common Miscon- Study Center for Anthropology, ceptions and Unproven Assumptions About Mechelbaan 338, 2580 Putte, the So-Called Aquatic Ape Hypothesis Belgium E-mail: [email protected] While some paleo-anthropologists remain skeptical, data from diverse biological and anthropological disciplines leave little doubt that human ancestors were at some point in our past semi- aquatic: wading, swimming and/or diving in shallow waters in search of waterside or aquatic foods. However, the exact sce- nario — how, where and when these semi-aquatic adaptations happened, how profound they were, and how they fit into the KEY WORDS: human evolution, hominid fossil record — is still disputed, even among anthro- Littoral theory, Aquarboreal pologists who assume some semi-aquatic adaptations. theory, aquatic ape, AAT, Here, I argue that the most intense phase(s) of semi-aquatic Archaic Homo, Homo erectus, adaptation in human ancestry occurred when populations be- Neanderthal, bipedalism, speech longing to the genus Homo adapted to slow and shallow littoral origins, Alister Hardy, Elaine diving for sessile foods such as shellfish during part(s) of the Morgan, comparative biology, Pleistocene epoch (Ice Ages), possibly along African or South- pachyosteosclerosis. Asian coasts. Introduction The term aquatic ape gives an incorrect impression of our semi-aquatic ancestors. Better terms are in my opinion the coastal dispersal model (Munro, 2010) or the littoral theory of human evolution, but although littoral seems to be a more appropriate biologi- cal term here than aquatic, throughout this paper I will use the well-known and common- ly used term AAH as shorthand for all sorts of waterside and semi-aquatic hypotheses. -

Australopiths Wading? Homo Diving?
Symposium: Water and Human Evolution, April 30th 1999, University Gent, Flanders, Belgium Proceedings Australopiths wading? Homo diving? http://allserv.rug.ac.be/~mvaneech/Symposium.html http://www.flash.net/~hydra9/marcaat.html Marc Verhaegen & Stephen Munro – 23 July 1999 Abstract Asian pongids (orangutans) and African hominids (gorillas, chimpanzees and humans) split 14-10 million years ago, possibly in the Middle East, or elsewhere in Eurasia, where the great ape fossils of 12-8 million years ago display pongid and/or hominid features. In any case, it is likely that the ancestors of the African apes, australopithecines and humans, lived on the Arabian-African continent 8-6 million years ago, when they split into gorillas and humans-chimpanzees. They could have frequently waded bipedally, like mangrove proboscis monkeys, in the mangrove forests between Eurasia and Africa, and partly fed on hard-shelled fruits and oysters like mangrove capuchin monkeys: thick enamel plus stone tool use is typically seen in capuchins, hominids and sea otters. The australopithecines might have entered the African inland along rivers and lakes. Their dentition suggests they ate mostly fruits, hard grass-like plants, and aquatic herbaceous vegetation (AHV). The fossil data indicates that the early australopithecines of 4-3 million years ago lived in waterside forests or woodlands; and their larger, robust relatives of 2-1 million years ago in generally more open milieus near marshes and reedbeds, where they could have waded bipedally. Some anthropologists believe the present-day African apes evolved from australopithecine-like ancestors, which would imply that knuckle-walking gorillas and chimpanzees evolved in parallel from wading- climbing ‘aquarborealists’.