Trigeminal Isolated Sensory Neuropathy (TISN) and FOSMN
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Hereditary Hypertrophic Neuropathy Combining Features of Tic Douloureux, Charcot- Marie-Tooth Disease, and Deafness
Hereditary hypertrophic neuropathy combining features of tic douloureux, Charcot- Marie-Tooth disease, and deafness Hereditary hypertrophic sensorimotor poly- neuropathy combining the features of Charcot- Marie-Tooth disease, trigeminal neuralgia, and Robert P. Cruse, D.O. deafness occurred through four generations of John P. Conomy, M.D. a family originating in Haywood County, North Asa J. Wilbourn, M.D. Carolina. Fourteen individuals had pes cavus, Maurice R. Hanson, M.D. distal muscle atrophy, depressed or absent mus- cle stretch reflexes, cutaneous sensory deficits, Department of Neurology and defective proprioception. Six family mem- bers had recurrent, lancinating, trigeminal pain, and seven were deaf. The family was brought under scrutiny when the propositus, a 60-year-old woman, was examined for treat- ment of tic douloureux. Neurologic informa- tion was ultimately obtained regarding 52 family members. No history of consanguinity could be ascertained within this kinship. The genealogy of the family we studied is presented in Figure 1. In addition to clinical neurologic examinations, electromyographic and nerve conduction stud- ies were obtained on those individuals indicated in the genealogy diagram. Audiometric studies were obtained when there was clinical indication of defective hearing. Quantitative cutaneous sensory testing was obtained in the propositus. 107 Downloaded from www.ccjm.org on October 1, 2021. For personal use only. All other uses require permission. 108 Cleveland Clinic Quarterly Vol. 44, No. 3 ' • CMT a TIC Deaf CMT He Examined MS f Died O Not Affected 0 D led before age onset (O Multiple normal d1 ond j si be. -1—i Two Marriages —Indicates number of slbs. -

Molecular Diagnostics of Charcot-Marie-Tooth Disease and Related Peripheral Neuropathies
17_Lupski 3/30/06 1:47 PM Page 243 NeuroMolecular Medicine Copyright © 2006 Humana Press Inc. All rights of any nature whatsoever reserved. ISSN0895-8696/06/08:243–254/$30.00 doi: 10.1385/NMM:8:1:243 REVIEW ARTICLE Molecular Diagnostics of Charcot-Marie-Tooth Disease and Related Peripheral Neuropathies Kinga Szigeti,1 Eva Nelis,2,3 and James R. Lupski*,1,4 1Departments of Molecular and Human Genetics, Baylor College of Medicine, Houston, TX; 2Molecular Genetics, Flanders Interuniversity Institute for Biotechnology, Institute Born-Bunge, University of Antwerp, Antwerpen, Belgium; 3Laboratory of Neurogenetics, Institute Born-Borge, University of Antwerp, Antwerpen, Belgium; and 4Pediatrics, Baylor College of Medicine, and Texas Children Hospital, Houston, TX 77030 Received January 10, 2006; Revised January 13, 2006; Accepted January 13, 2006 Abstract DNAdiagnostics plays an important role in the characterization and management of patients manifesting inherited peripheral neuropathies. We describe the clinical integration of molecular diagnostics with medical history, physical examination, and electrophysiological studies. Mole- cular testing can help establish a secure diagnosis, enable genetic counseling regarding recurrence risk, potentially provide prognostic information, and in the near future may be important for the choice of therapies. doi: 10.1385/NMM:8:1:243 Index Entries:Molecular diagnostics; Charcot-Marie-Tooth disease; CMT; hereditary neuropathy with liability to pressure palsies; HNPP; Dejerine-Sottas neuropathy; DSN; congenital hypomyelinating neuropathy; CHN; CMT1A duplication; HNPP deletion. Introduction genetic testing, one needs to be familiar with the diagnostic tests available, choose the appropriate Molecular genetic diagnosis has become an inte- patients for testing, and utilize the diagnostic tools gral part of the evaluation of patients with hered- in a logical fashion to optimize the use of resources. -

Neuropathic Orofacial Pain the Brochure Is Provided Compliments Of
Neuropathic_Pain_Brochure_Neuropathic_Pain_Brochure 6/9/2010 11:41 AM Page 1 Neuropathic orofacial paiN The brochure is provided compliments of This brochure in intended for informational purposes only and should be considered a replacement for a professional treatment for a health care professional. To locate knowledgeable and experienced expert in orofacial pain, contact: The American Academy of Orofacial Pain 174 S. New York Ave. POB 478 Oceanville, NJ 08231 P: 609-504-1311 E: [email protected] W: www.aaop.org To locate knowledgeable and experienced expert in Trigeminal Neuralgia, contact: Trigeminal Neuralgia Association 2801 SW Archer Road Gainesville, FL 32608 P: 352-376-9955 E: [email protected] W: www.tna-support.org Neuropathic_Pain_Brochure_Neuropathic_Pain_Brochure 6/9/2010 11:41 AM Page 3 coNteNts 1-Neuropathic orofacial paiN 4-GettiNG help/What to expect 6-commoN Neuropathic orofacial paiN DisorDers aND their treatmeNt 6 - triGem iNal NeuralGia 8 - pre-triGem iNal NeuralGia 9 - atypical oDoNtalGia (phaNtom tooth paiN) 10 - chroNic reGioNal paiN syNDrome 12 - iN coNclusioN Neuropathic_Pain_Brochure_Neuropathic_Pain_Brochure 6/9/2010 11:41 AM Page 4 Neuropathic orofacial paiN Of the many pains that can effect the head and neck, perhaps the most confusing and difficult to diagnose are a group of maladies called Neuropathic Orofacial Pain Disorders. These neuropathic pain disorders are often chronic and arise from the brain and nerves of the head, face and neck. If you have experienced the frustration of having a toothache or face pain and, after seeing many doctors, still don't know where the pain is coming from, Brain you may be suffering from a neuropathic pain Spinal Cord disorder. -

Trigeminal Neuralgia – Diagnosis and Treatment
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Archivio della ricerca- Università di Roma La Sapienza Review Cephalalgia 2017, Vol. 37(7) 648–657 ! International Headache Society 2017 Trigeminal neuralgia – Reprints and permissions: sagepub.co.uk/journalsPermissions.nav diagnosis and treatment DOI: 10.1177/0333102416687280 journals.sagepub.com/home/cep Stine Maarbjerg1, Giulia Di Stefano2, Lars Bendtsen1 and Giorgio Cruccu2 Abstract Introduction: Trigeminal neuralgia (TN) is characterized by touch-evoked unilateral brief shock-like paroxysmal pain in one or more divisions of the trigeminal nerve. In addition to the paroxysmal pain, some patients also have continuous pain. TN is divided into classical TN (CTN) and secondary TN (STN). Etiology and pathophysiology: Demyelination of primary sensory trigeminal afferents in the root entry zone is the predominant pathophysiological mechanism. Most likely, demyelination paves the way for generation of ectopic impulses and ephaptic crosstalk. In a significant proportion of the patients, the demyelination is caused by a neurovascular conflict with morphological changes such as compression of the trigeminal root. However, there are also other unknown etiological factors, as only half of the CTN patients have morphological changes. STN is caused by multiple sclerosis or a space-occupying lesion affecting the trigeminal nerve. Differential diagnosis and treatment: Important differential diagnoses include trigeminal autonomic cephalalgias, posttraumatic or postherpetic pain and other facial pains. First line treatment is prophylactic medication with sodium channel blockers, and second line treatment is neurosurgical intervention. Future perspectives: Future studies should focus on genetics, unexplored etiological factors, sensory function, the neurosurgical outcome and complications, combination and neuromodulation treatment as well as development of new drugs with better tolerability. -

1 United States District Court Eastern District Of
UNITED STATES DISTRICT COURT EASTERN DISTRICT OF TENNESSEE AT CHATTANOOGA JOHANNA DETERDING, ) ) Plaintiff, ) ) v. ) Case No:1:11-CV-13 ) Collier/Carter MICHAEL S. ASTRUE, ) Commissioner of Social Security, ) ) Defendant. ) REPORT AND RECOMMENDATION This action was instituted pursuant to 42 U.S.C. '' 405(g) and 1383(c)(3) seeking judicial review of the final decision of the Commissioner denying the plaintiff a period of disability, disability insurance benefits, and supplemental security income under Title II and Title XVI of the Social Security Act, 42 U.S.C. '' 416(I), 423, and 1382. 1 This matter has been referred to the undersigned pursuant to 28 U.S.C. ' 636(b) and Rule 72(b) of the Federal Rules of Civil Procedure for a report and recommendation regarding the disposition of plaintiff's motion for judgment on the pleadings (Doc. 11) and defendant=s motion for summary judgment (Doc. 17 ). For the reasons stated herein, I RECOMMENDED the Commissioner=s decision be REVERSED and REMANDED pursuant to Sentence Four of 42 U.S.C. ' 405(g). 1Since the relevant DIB and SSI regulations cited herein are virtually identical, citations will only be made to the DIB regulations, found at 20 C.F.R. '' 404.1500-404.1599. The parallel SSI regulations are found at 20 C.F.R. '' 416.900-416.999, corresponding to the last two digits of the DIB cites (e.g., 20 C.F.R. ' 404.1545 corresponds with 20 C.F.R. ' 416.945). 1 Case 1:11-cv-00013-CLC-WBC Document 19 Filed 02/02/12 Page 1 of 25 PageID #: <pageID> + Plaintiff's Age, Education, and Past Work Experience Plaintiff had a high-school education, along with three years of college, and was 40 years old at the time of her February 2009 hearing (Tr. -
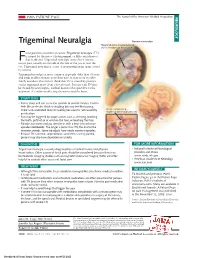
Trigeminal Neuralgia Trigeminal Neuralgia Trigeminal Nerve (Cranial Nerve V) and Its 3 Regions of Innervation Acial Pain Has a Number of Causes
JAMA PATIENT PAGE The Journal of the American Medical Association NEUROLOGY Trigeminal Neuralgia Trigeminal neuralgia Trigeminal nerve (cranial nerve V) and its 3 regions of innervation acial pain has a number of causes. Trigeminal neuralgia (TN) is named for the nerve (the trigeminal, or fifth cranial nerve) Fthat is affected. Trigeminal neuralgia causes brief, intense, BRAIN severe pain, usually on one side of the face or the jaw or near the V eye. Trigeminal neuralgia is a type of neuropathic pain (pain caused 1 by nerves). V V 2 Trigeminal neuralgia is more common in people older than 50 years 3 and tends to affect women more than men. It may occur in other family members. Researchers think that TN is caused by pressure on the trigeminal nerve from a blood vessel. Persons with TN may be treated by neurologists, medical doctors who specialize in the treatment of conditions affecting the nerves and the brain. BRAIN SYMPTOMS • Pain is sharp and can last a few seconds to several minutes. It often V feels like an electric shock or stabbing but may feel like burning. 1 There is no associated fever or swelling because it is not caused by Artery compressing trigeminal nerve and branches an infection. • Pain may be triggered by simple actions such as chewing, brushing V2 the teeth, puffs of air or wind on the face, or touching the face. V V 3 • Episodes can come and go, sometimes with a long time between 3 episodes (remission). The longer a person has TN, the shorter the V1 remission periods. -

A Misdiagnosis of Atypical Trigeminal Neuralgia
RESIDENT & FELLOW SECTION Clinical Reasoning: A misdiagnosis of atypical trigeminal neuralgia Jaclyn R. Duvall, MD, and Carrie E. Robertson, MD Correspondence Dr. Robertson Neurology 2019;93:124-131. doi:10.1212/WNL.0000000000007790 ® [email protected] Section 1 A 47-year-old man presented with right-sided facial pain that started 2 years prior. He described the pain as extremely intense, stabbing along the right jaw, lasting 5–60 seconds. This pain was exacerbated by chewing, and to a lesser degree, by brushing his teeth. The pain was so intense that he avoided eating when possible, leading to a 20-pound weight loss. When he did eat, he would try to chew on the left side of his mouth. Around the onset of these symptoms, he also noticed a persistent numbness and burning extending from the right lower earlobe to the lateral angle of the jaw that was exacerbated by turning his head to the right. The patient was given a diagnosis of atypical trigeminal neuralgia (TN) and sent to our headache clinic for further management. Questions for consideration: 1. What features are typical and atypical for classical TN? 2. What is your differential diagnosis in this patient presenting with facial pain? GO TO SECTION 2 From the Department of Neurology, Headache Division, Mayo Clinic, Rochester, MN. Go to Neurology.org/N for full disclosures. Funding information and disclosures deemed relevant by the authors, if any, are provided at the end of the article. 124 Copyright © 2019 American Academy of Neurology Copyright © 2019 American Academy of Neurology. Unauthorized reproduction of this article is prohibited. -

Osteopetrosis Associated with Familial Paraplegia: Report of a Family
Paraplegia (1975), 13, 143-152 OSTEOPETROSIS ASSOCIATED WITH FAMILIAL PARAPLEGIA: REPORT OF A FAMILY By SKIP JACQUES*, M.D., JOHN T. GARNER, M. D., DAVID JOHNSON, M.D. and C. HUNTER SHELDEN, M. D. Departments of Neurosurgery and Radiology, Huntington Memorial Hospital, Pasadena, Ca., and the Huntington Institute of Applied Medical Research, Pasadena, Ca., U.S.A. Abstract. A clinical analysis of three members of a family with documented osteopetrosis and familial paraplegia is presented. All patients had a long history of increased bone density and slowly progressing paraparesis of both legs. A thorough review of the literature has revealed no other cases which presented with paraplegia without spinal cord com pression. Although the etiologic factor or factors remain unknown, our review supports the contention that this is a distinct clinical entity. IN 1904, a German radiologist, Heinrich Albers-Schonberg, described a 26-year old man with multiple fractures and generalised sclerosis of the skeleton. The disease has henceforth commonly been known as Albers-Schonberg disease or marble osteopetrosis, a term first introduced by Karshner in 1922. Other eponyms are bone disease, osteosclerosis fragilis generalisata, and osteopetrosis generalisata. Approximately 300 cases had been reported in the literature by 1968. It has been generally accepted that the disease presents in two distinct forms, an infantile progressive disease and a milder form in childhood and adolescence. The two forms differ clinically and genetically. A dominant pattern of inheritance is usually seen in the benign type whereas the severe infantile form is usually inherited as a Mendelian recessive. This important distinction has not been well emphasised. -

Genetic Studies of Human Neuropathic Pain Conditions: a Review Katerina Zorina-Lichtenwalter*, Marc Parisien, Luda Diatchenko
NeuPSIG Reviews Genetic studies of human neuropathic pain conditions: a review Katerina Zorina-Lichtenwalter*, Marc Parisien, Luda Diatchenko Abstract Numerous studies have shown associations between genetic variants and neuropathic pain disorders. Rare monogenic disorders are caused by mutations of substantial effect size in a single gene, whereas common disorders are likely to have a contribution from multiple genetic variants of mild effect size, representing different biological pathways. In this review, we survey the reported genetic contributors to neuropathic pain and submit them for validation in a 150,000-participant sample of the U.K. Biobank cohort. Successfully replicated association with a neuropathic pain construct for 2 variants in IL10 underscores the importance of neuroimmune interactions, whereas genome-wide significant association with low back pain (P 5 1.3e-8) and false discovery rate 5% significant associations with hip, knee, and neck pain for variant rs7734804 upstream of the MAT2B gene provide evidence of shared contributing mechanisms to overlapping pain conditions at the molecular genetic level. Keywords: Neuropathic pain, Genetic association studies, Genetic variants, Single nucleotide polymorphisms, U.K. Biobank 1. Introduction During the past decade, the number of studies aiming to Neuropathic pain arises from a lesion or disease of the identify genetic factors in neuropathic pain conditions has grown somatosensory system.66 Although some conditions have in the hope of elucidating the molecular risk factors and identifying a known genetic cause, others develop as part of disease treatment targets. In this review, we summarise these studies and sequelae or posttraumatic complications. present the current landscape of neuropathic pain molecular The defining feature is an aberrant nociceptive network pathophysiology as it has been informed by them. -

The Effectiveness of Neurolytic Block of Sphenopalatine Ganglion Using
n e u r o l o g i a i n e u r o c h i r u r g i a p o l s k a 4 9 ( 2 0 1 5 ) 3 8 9 – 3 9 4 Available online at www.sciencedirect.com ScienceDirect journal homepage: http://www.elsevier.com/locate/pjnns Original research article The effectiveness of neurolytic block of sphenopalatine ganglion using zygomatic approach for the management of trigeminal neuropathy Malgorzata Malec-Milewska *, Bartosz Horosz, Dariusz Kosson, Agnieszka Sekowska, Hanna Kucia Pain Clinic: Department of Anesthesiology and Intensive Care, Medical Center for Postgraduate Education, Warsaw, Poland a r t i c l e i n f o a b s t r a c t Article history: This study was performed to present the outcomes of trigeminal neuropathy management Received 10 March 2015 with the application of neurolytic block of sphenopalatine ganglion. This type of procedure is Accepted 31 August 2015 used in cases where pain is not well controlled with medical treatment. Twenty patients Available online 19 September 2015 were treated with sphenopalatine ganglion neurolysis after their response to pharmacolog- ical management was not satisfactory. Significant pain relief was experienced by all but one Keywords: patient and they were able to reduce or stop their pain medication. The time of pain relief was between a few months and 9 years during the study period. Number of procedures Neuropathic pain implemented varied as some of the patients have been under the care of our Pain Clinic for as Trigeminal neuropathy long as 18 years, satisfied with this type of management and willing to have the procedure Sphenopalatine ganglion repeated if necessary. -
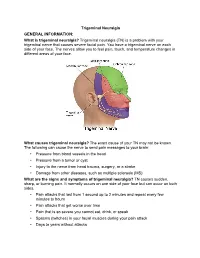
What Is Trigeminal Neuralgia? Trigeminal Neuralgia (TN) Is a Problem with Your Trigeminal Nerve That Causes Severe Facial Pain
Trigeminal Neuralgia GENERAL INFORMATION: What is trigeminal neuralgia? Trigeminal neuralgia (TN) is a problem with your trigeminal nerve that causes severe facial pain. You have a trigeminal nerve on each side of your face. The nerves allow you to feel pain, touch, and temperature changes in different areas of your face. What causes trigeminal neuralgia? The exact cause of your TN may not be known. The following can cause the nerve to send pain messages to your brain: • Pressure from blood vessels in the head • Pressure from a tumor or cyst • Injury to the nerve from head trauma, surgery, or a stroke • Damage from other diseases, such as multiple sclerosis (MS) What are the signs and symptoms of trigeminal neuralgia? TN causes sudden, sharp, or burning pain. It normally occurs on one side of your face but can occur on both sides. • Pain attacks that last from 1 second up to 2 minutes and repeat every few minutes to hours • Pain attacks that get worse over time • Pain that is so severe you cannot eat, drink, or speak • Spasms (twitches) in your facial muscles during your pain attack • Days to years without attacks What can trigger a pain attack? Most TN pain attacks are brought on by touching a trigger area on your face: • Eating or drinking • Smiling, yawning, or talking • Shaving or washing your face • Putting on makeup or combing your hair • Wind or temperature changes • Noise or lights How is trigeminal neuralgia diagnosed? Your caregiver will ask about your symptoms and when they started. Tell him if you have any close family members with TN. -

Neuropathic Pain a Management Update Milana Votrubec Ian Thong
Pain Neuropathic pain A management update Milana Votrubec Ian Thong Background Neuropathic pain is defined as ‘pain arising as a Neuropathic pain is described as burning, painful, cold or direct consequence of a lesion or disease affecting the electric shocks and may be associated with tingling, pins and somatosensory system’.1 This article will focus on the needles, numbness or itching. detection and management of diabetic polyneuropathy, Objective postherpetic neuralgia, trigeminal neuralgia and chronic This article summaries the diagnosis and management of four regional pain syndrome (CRPS). Importantly, disc disease common neuropathic pain presentations. and trauma can cause neuropathic pain, however these are Discussion beyond the scope of this article. A validated diagnostic screening tool can help identify patients with neuropathic pain. A systematic approach to A high index of suspicion is required for the diagnosis of neuropathic clinical assessment and investigation will clarify the diagnosis. pain as it can develop slowly over time. If neuropathic pain is suspected, Good glycaemic control is important in the prevention and a validated diagnostic screening tool such as the Leeds Assessment of management of diabetic polyneuropathy; management options Neuropathic Symptoms and Signs (LANSS), the Self reported LANSS include antidepressants, gabapentinoids and controlled release (S-LANSS), the Neuropathic Pain Questionnaire (NPQ), the Douleur opioids. Pain that lasts for more than 3 months after the onset Neuropathique en 4 (DN 4)