Using Static Method to Measure Tolmetin Solubility at Different
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

The Challenge of Drug-Induced Aseptic Meningitis Revisited
Letters cardioverter-defibrillator generator replacements and upgrade procedures: brospinal fluid (CSF) findings and reviews added to the litera- results from the REPLACE registry. Circulation. 2010;122(16):1553-1561. ture from 1999 to date. Tables have been assembled from 6. Kramer DB, Buxton AE, Zimetbaum PJ. Time for a change: a new approach to information derived from 192 studies (these data are avail- ICD replacement. N Engl J Med. 2012;366(4):291-293. able from the authors on request). The Challenge of Drug-Induced Aseptic Results | Four groups of drugs continue to be associated Meningitis Revisited with DIAM (Table 1): nonsteroidal anti-inflammatory Cases of drug-induced aseptic meningitis (DIAM) are likely drugs (NSAIDs), antibiotics, immunosuppressive- underreported, and only a few reviews of the literature have immunomodulatory (IS-IM), and antiepileptic drugs.1 Prior been performed. We have updated (to February 2014) a pre- exposure to the associated drug was present in 26% to 35% vious review (1999)1 to identify newer agents associated with of cases (Table 1). The interval between exposure and men- DIAM, as well as distinctive new features. ingitis ranged from minutes to 5 months (Table 1). Most patients presented with headache, fever, meningismus, and Methods | Using the MEDLINE database, we searched the lit- mental status changes (Table 2). Underlying systemic disor- erature to February 2014 and included those cases with cere- ders were often present, particularly systemic lupus ery- Table 1. Drugs Involved in Drug-Induced -

DAYPRO ALTA™ (Oxaprozin Potassium) 600Mg Tablets Cardiovascular Risk • Nsaids May Cause an Increased Risk of Serious Cardiov
DAYPRO ALTA™ (oxaprozin potassium) 600mg tablets Cardiovascular Risk NSAIDs may cause an increased risk of serious cardiovascular thrombotic events, myocardial infarction, and stroke, which can be fatal. This risk may increase with duration of use. Patient’s with cardiovascular disease or risk factors for cardiovascular disease may be at greater risk (see WARNINGS). Daypro ALTA is contraindicated for treatment of peri-operative pain in the setting of coronary artery bypass graft (CABG) surgery (see WARNINGS). Gastrointestinal Risk NSAID’s cause an increased risk of serious gastrointestinal adverse events including bleeding, ulceration, and perforation of the stomach or intestines, which can be fatal. These events can occur at any time during use and without warning symptoms. Elderly patients are at greater risk for serious gastrointestinal events (see WARNINGS). DESCRIPTION DAYPRO ALTA (oxaprozin potassium tablets) is a member of the propionic acid group of nonsteroidal anti-inflammatory drugs (NSAIDs). Each blue, capsule-shaped tablet contains oxaprozin potassium (678mg equivalent to 600mg of oxaprozin) for oral administration. The chemical name for oxaprozin potassium is 4,5-diphenyl-2-oxazolepropionic acid, potassium salt. Its empirical formula is C18H14NO3K, and molecular weight is 331. Oxaprozin potassium is a white to off white powder with a melting point of 215°C. It is slightly soluble in alcohol and very soluble in water. The PK in water is 9.7. It has the following structural formula: Inactive ingredients in DAYPRO ALTA tablets include microcrystalline cellulose, hydroxypropyl methylcellulose, pregelatinized corn starch, stearic acid, colloidal silicon dioxide, polyethylene glycol, titanium dioxide, FD&C Blue #1 Aluminum Lake, and pharmaceutical glaze. -

Mefenamic Acid Induced Autoimmune Hemolytic Anemia- a Case Report
The Journal of Medical Research 2020; 6(3): 67-69 Case Report Mefenamic acid induced autoimmune hemolytic anemia- A case JMR 2020; 6(3): 67-69 report May- June ISSN: 2395-7565 Reshmi Mishra1, Sarbeswar Pradhan2, Mahendravarma Madduri Reshmi Mishra3 © 2020, All rights reserved 1 Department of Pediatrics, Kalinga Institute of Medical Sciences (KIMS), Bhubaneswar- 751024, Odisha, India www.medicinearticle.com 2 Associate Professor, Department of Pediatrics, Kalinga Institute of Medical Sciences (KIMS), Bhubaneswar- Received: 15-05-2020 751024, Odisha, India Accepted: 13-06-2020 3 2nd Year PG, Department of Pediatrics, Kalinga Institute of Medical Sciences (KIMS), Bhubaneswar- 751024, Odisha, India Abstract Immune hemolytic anemia (IHA) is an uncommon adverse effect of a wide variety of drugs. The incidence is on the rise due to the extensive use of nonsteroidal anti-inflammatory drugs (NSAID). Mefenamic acid is the most common among NSAIDs causing AIHA by an autoimmune mechanism. We are presenting a case of three years old child presented with history dark colored urine, severe anemia, and jaundice after taking mefenamic acid for fever. Investigations revealed leucocytosis, hyperbilirubinemia, raised reticulocyte count, and negative direct Coombs test. The urinalysis demonstrated proteinuria, hematuria, and pus cell of 18-20/HPF. The urine for free hemoglobin was positive. As all the clinical, as well as investigation reports, were suggestive of the autoimmune hemolytic anemia except for the negative cooms test, the child was diagnosed to be a case of Coombs-negative autoimmune hemolytic anemia (Mefenamic induced) with urosepsis. He was managed conservatively by withdrawing the offending drug, and the latter steroid was added. -

Original Paper Enhancement of Chemotherapeutic Drug Toxicity To
European Journal of Cancer, Vol. 34, No. 8, pp. 1250±1259, 1998 # 1998 Elsevier Science Ltd. All rights reserved Pergamon Printed in Great Britain 0959-8049/98 $19.00+0.00 PII: S0959-8049(98)00045-8 Original Paper Enhancement of Chemotherapeutic Drug Toxicity to Human Tumour Cells In Vitro by a Subset of Non-steroidal Anti-in¯ammatory Drugs (NSAIDs) C.P. DuVy, C.J. Elliott, R.A. O'Connor, M.M. Heenan, S. Coyle, I.M. Cleary, K. Kavanagh, S. Verhaegen, C.M. O'Loughlin, R. NicAmhlaoibh and M. Clynes National Cell and Tissue Culture Centre, Dublin City University, Glasnevin, Dublin 9, Ireland The eVect on cytotoxicity of combining a range of clinically important non-steroidal anti-in¯amma- tory drugs (NSAIDs) with a variety of chemotherapeutic drugs was examined in the human lung cancer cell lines DLKP, A549, COR L23P and COR L23R and in a human leukaemia line HL60/ADR. A speci®c group of NSAIDs (indomethacin, sulindac, tolmetin, acemetacin, zomepirac and mefenamic acid) all at non-toxic levels, signi®cantly increased the cytotoxicity of the anthracyclines (doxorubicin, daunorubicin and epirubicin), as well as teniposide, VP-16 and vincristine, but not the other vinca alkaloids vinblastine and vinorelbine. Asubstantial number of other anticancer drugs, including methotrexate, 5-¯uorouracil, cytarabine, hydroxyurea, chlorambucil, cyclophosphamide, cisplatin, carboplatin, mitoxantrone, actinomycin D, bleomycin, paclitaxel and camptothecin, were also tested, but displayed no synergy in combination with the NSAIDs. The synergistic eVect was concentration dependent. The eVect appears to be independent of the cyclo-oxygenase inhibitory ability of the NSAIDs, as (i) the synergistic combination could not be reversed by the addition of prostaglandins D2 or E2; (ii) sulindac sulphone, a metabolite of sulindac that does not inhibit the cyclooxygenase enzyme, was positive in the combination assay: and (iii) many NSAIDs known to be cyclo-oxygenase inhibitors, e.g. -

Formulary Drug Listing Decisions PIROXICAM
March 1, 2010 Formulary Drug Listing Decisions PIROXICAM Indication (s) of chronic arthritis (e.g., osteoarthritis, rheumatoid arthritis and ankylosing Piroxicam is used to treat pain and a spondylitis). This labeling change for variety of inflammatory conditions. piroxicam is relevant for the WSIB population, many of whom are pre- DAC Recommendation scribed NSAIDs for treatment of acute, The Drug Advisory Committee (DAC) has short-term pain. recommended that piroxicam be removed Piroxicam was cited as having an from all WSIB formularies following a increased risk of serious skin reactions Health Canada safety review that cited (e.g., Stevens-Johnson syndrome and piroxicam’s, acute, short-term increased toxic epidermal necrolysis) and gas- risk of serious skin conditions and trointestinal problems relative to other gastrointestinal intestinal (GI) problems NSAIDs. compared to other nonsteroidal anti- Drug Profile inflammatory drugs (NSAIDs). There are a number of equally effective NSAIDs (e.g., ibuprofen, diclofenac) that Products available can be used for treatment of inflamma- in Canada: The WSIB Decision tory conditions, both on an acute and Apo-Piroxicam, Based on the DAC’s recommendations, the chronic basis. Dom-Piroxicam, WSIB has decided to remove piroxicam Gen-Piroxicam, from all formularies at this time. The DAC recommended that piroxicam Novo-Pirox, Nu-Pirox, be removed from WSIB formularies PMS-Piroxicam, Formulary Status based on a Health Canada review of Pro-Piroxicam studies suggesting an increase in GI MANUFACTURER: Variety Piroxicam HAS BEEN REMOVED from toxicity and an increased risk of devel- of generic manufac- WSIB formularies at this time. opment of serious skin reactions (which turers has resulted in labeling changes by Recommendation Highlights Health Canada) and on the availability of Piroxicam is a non-selective NSAID that equally effective NSAIDs for treatment has been used in the treatment of pain of inflammatory conditions. -

Nonsteroidal Anti-Inflammatory Drug Use Among Persons with Chronic Kidney Disease in the United States
Nonsteroidal Anti-Infl ammatory Drug Use Among Persons With Chronic Kidney Disease in the United States 1,2 Laura Plantinga, ScM ABSTRACT 1 Vanessa Grubbs, MD PURPOSE Because avoidance of nonsteroidal anti-infl ammatory drugs (NSAIDs) is Urmimala Sarkar, MD1,2 recommended for most individuals with chronic kidney disease (CKD), we sought to characterize patterns of NSAID use among persons with CKD in the United States. Chi-yuan Hsu, MD1 METHODS A total of 12,065 adult (aged 20 years or older) participants in the 3 Elizabeth Hedgeman, MS cross-sectional National Health and Nutrition Examination Survey (1999-2004) Bruce Robinson, MD4 responded to a questionnaire regarding their use of over-the-counter and pre- 3 scription NSAIDs. NSAIDs (excluding aspirin and acetaminophen) were defi ned by Rajiv Saran, MD self-report. CKD was categorized as no CKD, mild CKD (stages 1 and 2; urinary Linda Geiss, MS5 albumin-creatinine ratio of ≥30 mg/g) and moderate to severe CKD (stages 3 and 4; estimated glomerular fi ltration rate of 15-59 mL/min/1.73 m2). Adjusted MPH5 Nilka Ríos Burrows, prevalence was calculated using multivariable logistic regression with appropriate Mark Eberhardt, PhD6 population-based weighting. 1,2 Neil Powe, MD RESULTS Current use (nearly every day for 30 days or longer) of any NSAID was For the CDC CKD Surveillance reported by 2.5%, 2.5%, and 5.0% of the US population with no, mild, and Team moderate to severe CKD, respectively; nearly all of the NSAIDs used were avail- able over-the-counter. Among those with moderate to severe CKD who were 1 Department of Medicine, University of currently using NSAIDs, 10.2% had a current NSAID prescription and 66.1% had California, San Francisco, California used NSAIDs for 1 year or longer. -

Indocin® (Indomethacin) Oral Suspension
INDOCIN® (INDOMETHACIN) ORAL SUSPENSION Cardiovascular Risk • NSAIDs may cause an increased risk of serious cardiovascular thrombotic events, myocardial infarction, and stroke, which can be fatal. This risk may increase with duration of use. Patients with cardiovascular disease or risk factors for cardiovascular disease may be at a greater risk. (See WARNINGS.) • INDOCIN is contraindicated for the treatment of peri-operative pain in the setting of coronary artery bypass graft (CABG) surgery (see WARNINGS). Gastrointestinal Risk • NSAIDs cause an increased risk of serious gastrointestinal adverse events including bleeding, ulceration, and perforation of the stomach or intestines, which can be fatal. These events can occur at any time during use and without warning symptoms. Elderly patients are at greater risk for serious gastrointestinal events. (See WARNINGS.) DESCRIPTION Suspension INDOCIN1 for oral use contains 25 mg of indomethacin per 5 mL, alcohol 1%, and sorbic acid 0.1% added as a preservative and the following inactive ingredients: antifoam AF emulsion, flavors, purified water, sodium hydroxide or hydrochloric acid to adjust pH, sorbitol solution, and tragacanth. Indomethacin is a non-steroidal anti-inflammatory indole derivative designated chemically as 1-(4-chlorobenzoyl)-5-methoxy-2-methyl-1H-indole-3-acetic acid. 1 Indomethacin is practically insoluble in water and sparingly soluble in alcohol. It has a pKa of 4.5 and is stable in neutral or slightly acidic media and decomposes in strong alkali. The suspension has a pH of 4.0-5.0. The structural formula is: 1 Registered trademark of MERCK & CO., Inc., Whitehouse Station, NJ U.S.A. and licensed to Iroko Pharmaceuticals, LLC, Philadelphia, PA, U.S.A. -

Nsaids Separation on HALO 2 ES-CN with MS Compatible Mobile Phase
Application Note: 128-NS NSAIDs Separation on HALO 2 ES-CN with MS Compatible Mobile Phase 3 G0112 1 PEAK IDENTITIES: 5 1. Aspirin 2. Tolmetin 3. Naproxen 4. Fenoprofen 2 5. Ibuprofen 6. Diclofenac 4 6 Absorbance @ 230 nm @ 230 Absorbance 0.0 0.2 0.4 0.6 0.8 1.0 Minutes TEST CONDITIONS: STRUCTURES: Column: 3.0 x 50 mm, HALO 2 ES-CN Part Number: 91813-404 Mobile Phase: 60/40-A/B A= Water with 0.1% formic acid/ 10 mM ammonium formate, pH 3.3 B= 80/20 Acetonitrile/Water with 0.1% formic acid/10 mM ammonium formate Flow Rate: 2.0 mL/min. Pressure: 440 bar Aspirin Naproxen Ibuprofen Temperature: 45 °C Detection: UV 230 nm, PDA Injection Volume: 1 μL Sample Solvent: Water/methanol Data Rate: 80 Hz Response Time: 0.02 sec. Flow Cell: 2 μL micro cell LC System: Agilent 1200 SL Tolmetin Fenoprofen Diclofenac Six NSAIDS are separated on a HALO 2 ES -CN column using a MS compatible mobile phase in less than 1 minute with symmetrical peak shape s. FOR MORE INFORMATION OR TO PLACE AN ORDER, CONTACT: www.advanced-materials-tech.com ® HALO and Fused-Core are registered trademarks of Advanced Materials Technology, Inc. Application Note: 074-NS Separation of NSAIDs on HALO-5 and Totally Porous 5 µm 3 G0057 1 HALO-5 C18 4 5 PEAK IDENTITIES: 2 1. Acetaminophen 6 8 9 7 2. Aspirin 3. Salicylic acid 4. Tolmetin 3 1 STRUCTUR 5. Ketoprofen Totally Porous 5 µm C18 6. -
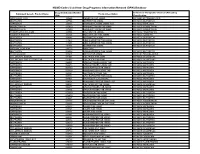
NSAID Codes Used from Drug Programs Information Network (DPIN) Database
NSAID Codes Used from Drug Programs Information Network (DPIN) Database Drug Identification Number Anatomical Therapeutic Chemical (ATC) Drug Equivalent Generic Product Name Product Description (DIN) Classification MEFENAMIC ACID 155225 PONSTAN CAP 250MG M01AG01 MEFENAMIC ACID IBUPROFEN 327794 MOTRIN TAB 300MG M01AE01 IBUPROFEN NAPROXEN 335193 NAPROSYN 250MG 250MG TAB M01AE02 NAPROXEN INDOMETHACIN 337420 NOVO-METHACIN CAP 25MG M01AB01 INDOMETACIN INDOMETHACIN 337439 NOVO-METHACIN CAP 50MG M01AB01 INDOMETACIN FENOPROFEN CALCIUM 345504 NALFON TAB 600MG M01AE04 FENOPROFEN TOLMETIN SODIUM 364126 TOLECTIN 200 TAB 200MG M01AB03 TOLMETIN IBUPROFEN 364142 MOTRIN TAB 400MG M01AE01 IBUPROFEN IBUPROFEN 441643 APO IBUPROFEN TAB 200MG M01AE01 IBUPROFEN IBUPROFEN 441651 APO IBUPROFEN TAB 300MG M01AE01 IBUPROFEN IBUPROFEN 484911 MOTRIN TAB 600MG M01AE01 IBUPROFEN NAPROXEN SODIUM 491772 ANAPROX IBUPROFEN 506052 APO-IBUPROFEN TAB 400MG M01AE01 IBUPROFEN PENICILLAMINE 511641 DEPEN TAB 250MG M01CC01 PENICILLAMINE DICLOFENAC SODIUM 514004 VOLTAREN TAB 25MG M01AB05 DICLOFENAC DICLOFENAC SOD SR 75MG TAB 514012 VOLTAREN TAB 50MG M01AB05 DICLOFENAC NAPROXEN 522651 APO NAPROXEN 250MG TAB M01AE02 NAPROXEN NAPROXEN 522678 APO NAPROXEN 125MG TAB M01AE02 NAPROXEN NAPROXEN 565350 NOVO-NAPROX TAB 250MG M01AE02 NAPROXEN NAPROXEN 565369 NOVO-NAPROX 125MG TAB M01AE02 NAPROXEN NAPROXEN 583367 NAPROSYN 375MG TAB M01AE02 NAPROXEN IBUPROFEN 585114 APO IBUPROFEN TAB 600MG M01AE01 IBUPROFEN NAPROXEN 587923 NAPROSYN SUS 25MG/ML M01AE02 NAPROXEN NAPROXEN 589861 NOVO-NAPROX TAB -

ERX.NPA.20 Celecoxib (Celebrex)
Clinical Policy: Celecoxib (Celebrex) Reference Number: ERX.NPA.20 Effective Date: 06.01.15 Last Review Date: 08.17 Line of Business: Commercial [Prescription Drug Plan] Revision Log See Important Reminder at the end of this policy for important regulatory and legal information. Description Celecoxib (Celebrex®) is a nonsteroidal anti-inflammatory drug (NSAID). FDA approved indication Celebrex is indicated: For the treatment of osteoarthritis For the treatment of rheumatoid arthritis For the treatment of juvenile rheumatoid arthritis in patients 2 years and older For the treatment of ankylosing spondylitis For the treatment of acute pain For the treatment of primary dysmenorrhea Policy/Criteria Provider must submit documentation (which may include office chart notes and lab results) supporting that member has met all approval criteria It is the policy of health plans affiliated with Envolve Pharmacy Solutions™ that Celebrex is medically necessary when the following criteria are met: I. Initial Approval Criteria A. All Indications (must meet all): 1. Age ≥ 2 years; 2. Member meets one of the following (a, b, c, d, or e): a. Age > 65 years; b. Current use of corticosteroid; c. Current use of an anticoagulant (e.g., aspirin, warfarin, low molecular weight heparin, direct thrombin inhibitors, factor Xa inhibitors, clopidogrel); d. Prior gastrointestinal bleed or active peptic ulcer disease (not gastroesophageal reflux disease [GERD]); e. Both of the following (i and ii): i. Failure of a ≥ 4 week trial of meloxicam at up to maximally indicated doses unless contraindicated or clinically significant adverse effects are experienced; ii. Failure of a ≥ 4 week trial of one additional generic NSAID at up to maximally indicated doses unless contraindicated or clinically significant adverse effects are experienced; 3. -

Plants As Sources of Anti-Inflammatory Agents
molecules Review Plants as Sources of Anti-Inflammatory Agents Clara dos Reis Nunes 1 , Mariana Barreto Arantes 1, Silvia Menezes de Faria Pereira 1, Larissa Leandro da Cruz 1, Michel de Souza Passos 2, Luana Pereira de Moraes 1, Ivo José Curcino Vieira 2 and Daniela Barros de Oliveira 1,* 1 Laboratório de Tecnologia de Alimentos, Centro de Ciências e Tecnologias Agropecuárias, Universidade Estadual do Norte Fluminense Darcy Ribeiro, Campos dos Goytacazes, RJ 28013-602, Brazil; [email protected] (C.d.R.N.); [email protected] (M.B.A.); [email protected] (S.M.d.F.P.); [email protected] (L.L.d.C.); [email protected] (L.P.d.M.) 2 Laboratório de Ciências Químicas, Centro de Ciências e Tecnologia, UniversidadeEstadual do Norte Fluminense Darcy Ribeiro, Campos dos Goytacazes, RJ 28013-602, Brazil; [email protected] (M.d.S.P.); [email protected] (I.J.C.V.) * Correspondence: [email protected]; Tel.: +55-22-988395160 Academic Editors: Thea Magrone, Rodrigo Valenzuela and Karel Šmejkal Received: 29 June 2020; Accepted: 5 August 2020; Published: 15 August 2020 Abstract: Plants represent the main source of molecules for the development of new drugs, which intensifies the interest of transnational industries in searching for substances obtained from plant sources, especially since the vast majority of species have not yet been studied chemically or biologically, particularly concerning anti-inflammatory action. Anti-inflammatory drugs can interfere in the pathophysiological process of inflammation, to minimize tissue damage and provide greater comfort to the patient. Therefore, it is important to note that due to the existence of a large number of species available for research, the successful development of new naturally occurring anti-inflammatory drugs depends mainly on a multidisciplinary effort to find new molecules. -

Celecoxib) Prior Authorization Request Form DO NOT COPY for FUTURE USE
OptumRx has partnered with CoverMyMeds to receive prior authorization requests, rex saving you time and often delivering real-time determinations. Visit go.covermymeds.com/OptumRx to begin using this free service. Please note: All information below is required to process this request. Mon-Fri: 5am to 10pm Pacific / Sat: 6am to 3pm Pacific Celebrex® (celecoxib) Prior Authorization Request Form DO NOT COPY FOR FUTURE USE. FORMS ARE UPDATED FREQUENTLY AND MAY BE BARCODED Member Information (required) Provider Information (required) Member Name: Provider Name: Insurance ID#: NPI#: Specialty: Date of Birth: Office Phone: Street Address: Office Fax: City: State: Zip: Office Street Address: Phone: City: State: Zip: Medication Information (required) Medication Name: Strength: Dosage Form: Check if requesting brand Directions for Use: Check if request is for continuation of therapy Clinical Information (required) Select the diagnosis below: Acute pain Ankylosing spondylitis Juvenile rheumatoid arthritis Osteoarthritis (OA) Primary dysmenorrhea Rheumatoid arthritis (RA) Other diagnosis: ______________________________ ICD-10 Code(s): _____________________________________ Select the medications the patient has had a history of: Diclofenac potassium Ibuprofen Nabumetone Diclofenac sodium Indomethacin Naproxen Etodolac Ketoprofen Oxaprozin Etodolac ER Ketorolac Piroxicam Fenoprofen Meclofenamate Sulindac Flurbiprofen Meloxicam Tolmetin Other Nonsteroidal Anti-inflammatory Drugs (NSAIDs). Please specify:___________________________________________________