Lone Pairs Are of Equal Importance and These Distribute Themselves to Minimize Interelectron Repulsion Problems, for Example, with the Deviation
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

No Rabbit Ears on Water. the Structure of the Water Molecule
No Rabbit Ears on Water The Structure of the Water Molecule: What Should We Tell the Students? Michael Laing University of Natal. Durban, South Africa In the vast majority of high school (I)and freshman uni- He also considers the possibility of s character in the bond- versity (2) chemistry textbooks, water is presented as a bent ing orbitals of OF2 and OH2 to improve the "strength" of the molecule whose bonding can be described bv the Gilles~ie- orbital (p 111) from 1.732 for purep to 2.00 for the ideal sp3. Nyholm approach with the oxygen atomsp3 Gyhridized. The Inclusion of s character in the hybrid will be "resisted in the lone uairs are said to he dominant causine the H-O-H anele case of atoms with an nnshared pair.. in the s orbital, to beieduced from the ideal 1091120to 104' and are regul&ly which is more stable than the p orbitals" (p 120). Estimates shown as equivalent in shape and size even being described of the H-E-H angle range from 91.2 for AsH3 to 93.5O for as "rabbit ears" (3). Chemists have even been advised to Hz0. He once again ascribes the anomalies for Hz0 to "re- wear Mickey Mouse ears when teaching the structure and uulsion of the charzes of the hvdroaeu atoms" (D 122). bonding of the H20molecule, presumably to emphasize the - In his famous hook (6)~itac~oro&kii describks the bond- shape of the molecule (and possibly the two equivalent lone ing in water and HzS (p 10). -
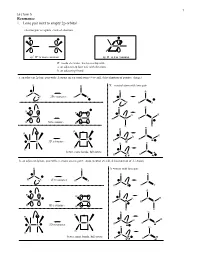
Resonance 1. Lone Pair Next to Empty 2P Orbital
1 Lecture 5 Resonance 1. Lone pair next to empty 2p orbital electron pair acceptors - lack of electrons C C CC sp2 R+ is more common sp R+ is less common R+ needs electrons, has to overlap with a. an adjacent 2p lone pair with electrons b. an adjacent pi bond a. an adjacent 2p lone pair with electrons on a neutral atom (+ overall, delocalization of positive charge) R R X = neutral atom with lone pair R R C C R X 2D resonance R X C C R X R X R R R R C R C R CX CX R N R N R 3D resonance R R R R R R R C R C R CX R O CX 3D resonance R O R R R R C better, more bonds, full octets C R F R F b. an adjacent 2p lone pair with electrons on a negative atom (neutral overall, delocalization of electrons) R R X =anion with lone pair R R C C R X 2D resonance R X C C R X R X R R R R C R C R CX CX R C R C R 3D resonance R R R R R R R C R C R CX R N CX 3D resonance R N R R R R C C better, more bonds, full octets R O R O 2 Lecture 5 Problem 1 – All of the following examples demonstrate delocalization of a lone pair of electrons into an empty 2p orbital. Usually in organic chemistry this is a carbocation site, but not always. -

Effect of Lone Pairs on Hybridization
EFFECT OF LONE PAIRS ON HYBRIDIZATION We have discussed the effect of ghost lone pairs on the shape of a molecule in the post about VSEPR theory. The effect lone pairs exert is the same in VBT as it was in VSEPR. In this post we will study a few examples in which central atom possess lone pairs. First, we will take the example of NH3 molecule. When you draw its Lewis dot structure, you will find three bonding pairs of electrons and one lone pair on N. Now write the ground state configuration of N. 7N: 1s2, 2s2, 2p3 N already has 3 unpaired electrons in ground state to make bond with 3 H atoms. You may think why does N need hybridization in this case when it has 3 unpaired electrons of the same orbital p? Because hybridized orbitals are more competent in overlapping which results in a stronger bond. 3 In NH3 molecule, N chooses sp hybridization. This may get you puzzled again, if N has 3 unpaired electrons in p why does it include s orbital which has paired electrons? You know that the atoms follow Hund’s rule for filling electrons in orbitals, similarly they have to consider energy order of orbitals in hybridization also. When an atom undergoes hybridization, it has to pick orbitals in the increasing order of their energies. In NH3, N has to first pick 2s then 2px, 2py and then 2pz. N can’t ignore 2s even if it is filled because 2s has the lowest energy and N has to include all three 2p orbitals because it needs them for bonding with three H atoms. -

How Do We Know That There Are Atoms
Chemistry 11 Fall 2010 Discussion – 12/13/10 MOs and Hybridization 1. a. [:C N:]- Bond order = (8 – 2) / 2 = 3 *2p * 2p C 2p N 2p 2p 2p *2s C 2s N 2s 2s CN- b. Comparison of Lewis and MO models of CN- CN- Consistent: Both have triple bond (B.O. = 3) Inconsistent: - Lewis structure shows lone pairs on each atom rather than all e- being shared (MO) - Lewis structure indicates a negative formal charge on C, whereas MO suggests more electron density on N, which is consistent with EN considerations c. Both molecules exhibit sp mixing, and the MOs should reflect this. In N2, the molecular orbitals would be distributed equally between the two N atoms, whereas in CN-, the bonding orbitals have greater electron density on the more electronegative atom (N). The antibonding orbitals have more electron density on the less electronegative atom (C). - 1 - Chemistry 11 Fall 2010 2. a. Completed structure of guanine (carbons at each intersection are implied): * * * * b. 17 sigma bonds; 4 pi bonds. c. All carbons are sp2 hybridized; trigonal planar; with 120˚ bond angles. d. We predict that the three N’s marked with * are sp3 hybridized, with 109.5˚ bond angles. The remaining N atoms are sp2 hybridized, with 120˚ bond angles. e. i. Benzene: ii. For the structure drawn in (a), the bond angles in the six membered ring would be expected to be 120˚ for each atom except for the N§, which is predicted to have bond angles of 109.5˚. However, it is not possible to form a planar six-membered ring unless ALL the angles are 120˚. -

Localized Electron Model for Bonding What Does Hybridization Mean?
190 Localized electron model for bonding 1. Determine the Lewis Structure for the molecule 2. Determine resonance structures 3. Determine the shape of the molecule 4. Determine the hybridization of the atoms What does hybridization mean? Why hybridize? Look at the shape of many molecules, and compare this to the shape of the orbitals of each of the atoms. Shape of orbitals Orbitals on atoms are s, p, d, and f, and there are certain shapes associated with each orbital. an s orbital is a big sphere the f orbitals look like two four leaf the p orbitals looks like “8” ’s clovers each one points in along an axis three f orbitals look like weird “8”’s with two doughnuts around each four of the d orbitals look like four one leaf clovers one d orbital looks like an “8” with a doughnut around the middle Shape of molecules Remember we determine the shape of molecules by determining the number of sets of electrons around the atom. count double and triple bonds as one set, count single bonds as one set of electrons and count lone pairs as a set 2 sets—each pair of electrons points 180° away from the other 5 sets—each pair points toward the corner of a trigonal bipyramid 3 sets—each pair points toward the corner of a triangle 6 sets—each pair points toward the corners of an octahedron 4 sets—each pair points toward the corner of a tetrahedron 191 To make bonds we need orbitals—the electrons need to go some where. -

Origin of Chalcogen-Bonding Interactions
Edinburgh Research Explorer The Origin of Chalcogen-Bonding Interactions Citation for published version: Pascoe, D, Ling, K & Cockroft, S 2017, 'The Origin of Chalcogen-Bonding Interactions', Journal of the American Chemical Society, vol. 139, no. 42, pp. 15160–15167. https://doi.org/10.1021/jacs.7b08511 Digital Object Identifier (DOI): 10.1021/jacs.7b08511 Link: Link to publication record in Edinburgh Research Explorer Document Version: Peer reviewed version Published In: Journal of the American Chemical Society General rights Copyright for the publications made accessible via the Edinburgh Research Explorer is retained by the author(s) and / or other copyright owners and it is a condition of accessing these publications that users recognise and abide by the legal requirements associated with these rights. Take down policy The University of Edinburgh has made every reasonable effort to ensure that Edinburgh Research Explorer content complies with UK legislation. If you believe that the public display of this file breaches copyright please contact [email protected] providing details, and we will remove access to the work immediately and investigate your claim. Download date: 11. Oct. 2021 The Origin of Chalcogen-Bonding Interactions Dominic J. Pascoe†, Kenneth B. Ling‡, and Scott L. Cockroft†* †EaStCHEM School of Chemistry, University of Edinburgh, Joseph Black Building, David Brewster Road, Edinburgh, EH9 3FJ, UK ‡Syngenta, Jealott’s Hill International Research Centre, Bracknell, Berkshire, RG42 6EY, UK ABSTRACT: Favorable molecular interactions between group 16 elements have been implicated in catalysis, biological processes, materials and medicinal chemistry. Such interactions have since become known as chalcogen bonds by analogy to hydrogen and halogen bonds. -

Exam 1 Parts I and II Answers
ORGANIC CHEMISTRY FALL 2019 LANEY COLLEGE CHEM 12A (L1/L1LA/L1LB) INSTRUCTOR: STEPHEN CORLETT EXAM 1, Part II (150 points) Page 1 of 5 Name ___________________________________________ Key ORGANIC CHEMISTRY FALL 2019 LANEY COLLEGE CHEM 12A (L1/L1LA/L1LB) INSTRUCTOR: STEPHEN CORLETT EXAM 1, Part II (150 points) Page 2 of 5 1. The compound called DMAP is shown below. a. Indicate the hybridization for each of the nitrogen atoms. b. Identify what type of orbital each of the lone pair of electrons is in. c. Indicate whether each lone pair of electrons is localized or delocalized. (15 points) a ~ . a lone pair - is in a orbital 2 p ← 2 and delocalized go 2 ← lone pair is go 2$ in an spa orbital and is localized 2. For the structure shown below, push electrons to show the other resonance form. Circle the major resonance form and provide the reason for your choice. (15 points) " " o? - . f ¥7man. 3. Consider the three compounds below. Indicate which compound has the largest pKa and which has the smallest pKa. Use ARIO to justify your answers (don’t forget about the conj. bases) (20 points) smallest middle pka largest pka pka O O O It H H ① ~ o_O →NH 150 Is more least stable most stable since oxygen than element is electronegative 3rd row and more able N so better able to , ingest a -0 support -0 charge to support charge ORGANIC CHEMISTRY FALL 2019 LANEY COLLEGE CHEM 12A (L1/L1LA/L1LB) INSTRUCTOR: STEPHEN CORLETT EXAM 1, Part II (150 points) Page 3 of 5 4. -

STEREOCHEMISTRY and ORDERING in the TETRAHEDRAL Portion of SILICATES G. E. Bnownl and G. V. Grses, Department of Geological Scie
THE AMERTCAN MINDRALOGISI, VOL 55, SEPTEMBER-OCTOBER' 1970 STEREOCHEMISTRY AND ORDERING IN THE TETRAHEDRAL PORTiON OF SILICATES G. E. BnowNl aND G. V. Grses, Departmentof GeologicalScieruces, Virginia PolytechnicInstitute ortd State Llniaersity, Blocksbwrg,Virginia 24061 ABSi RAcr The depentlence of si-o(br) and Si-o(nbr) bonrl lengths on Si-o si angle and average electronegativity,v, oI non-tetrahedral cations has been examined for four c2/rn anphi- boles and a number of other silicates with both bridging, O(br), and non-bridging, O(nbr), oxvgens. When both 1 and Si O-Si angle are large, the mean Si O(nbr) bond lengths, <Si-O(nbr)), are observed to be longer than the mean SiO(br) bond lengths, {Si-O (br)). Horn,ever,when i is small, <Si-O(nbr)> are usually shorter than or equal to <Si-O(br)>, irrespective of angle. A correlation oi (T-O) bond length (T=Si,Al,P) with o. o non-bonded distance in o-?-o linkages for compounds with sior as well as Alor and Por tetrahedra is consistent with cruickshank's (1961) r-bonding hypothesis, i,vith McDonald and Cruickshank's (1967) assumption that O(nbr) have larger negative chargesthan O(br) and with Gillespie's (1963) VSEPR Theory. The distributions of tetrahedral Si and A1, B, Be or Mg in a number of framervork sili- cates are examined in terms of Cruickshank's hypothesis and the difference in predicted si-o, Ai o, B-o, Be o and Mg-o bond orders for zo4"- tetrahedral ions and are found to be consistent with the proposal that Si should prefer those tetrahedra involved in the rvidest average t-O-t angles. -

Visualizing Lone Pairs in Compounds Containing Heavier Congeners of the Carbon and Nitrogen Group Elements
Proc. Indian Acad. Sci. (Chem. Sci.), Vol. 113, Nos 5 & 6, October–December 2001, pp 487–496 Ó Indian Academy of Sciences Visualizing lone pairs in compounds containing heavier congeners of the carbon and nitrogen group elements RAM SESHADRI Solid State and Structural Chemistry Unit, Indian Institute of Science, Bangalore 560 012, India e-mail: [email protected] Abstract. In this mini-review, I discuss some recent work on the stereochemistry and bonding of lone pairs of electrons in divalent compounds of the heavier carbon group elements (SnII, PbII) and in trivalent compounds of the heavier nitrogen group elements (BiIII). Recently developed methods that permit the real-space visualization of bonding patterns on the basis of density functional calculations of electronic structure, reveal details of the nature of s electron lone pairs in compounds of the heavier main group elements – their stereochemistry and their inertness (or lack thereof). An examination of tetragonal P4/nmm SnO, a-PbO and BiOF, and cubic Fm3m PbS provides a segue into perovskite phases of technological significance, including ferroelectric PbTiO3 and antiferroelectric/piezoelectric PbZrO3, in both of which the lone pairs on Pb atoms play a pivotal rôle. Keywords. Main group elements; lone pairs; stereochemistry; ferroelectricity. 1. Introduction The valence shell electron pair repulsion (VSEPR) theory, first suggested by Sidgwick and Powell 1, to be refined later by Gillespie and Nyholm 2, is the stuff of high school chemistry. The sequence of decreasing repulsion, lone pair–lone pair > lone pair–bond pair > bond pair–bond pair, is a familiar thumb rule that permits thousands of school and college students to hazard the geometry of molecules such as XeO3. -

Overview of Chapters 10 & 11 Theory of Covalent Bonding
Overview of Chapters 10 & 11 Theory of Covalent Bonding Starting with the molecular formula for a covalently bonded molecule or polyatomic ion it is possible to predict a wide range of molecular properties by applying a few simple procedures. These include drawing the Lewis structure to determine the distribution of electrons about the central atom, applying the VSEPR theory to predict the electron geometry and molecular shape of the molecule, using formal charges to predict the most probable resonance structure for a molecule if there are multiple ones to choose from, and using electronegativity values to predict molecular polarity. The electron geometry can also be used to predict the hybridization of the atomic orbitals and the types of covalent bonds (σ and π) that are used to bond the atoms together. Below is an example of apply these procedures to a molecule of COBr2 A. Draw the Lewis structure (Figure 10.1) 1. Step 1: The carbon has the lowest electronegativity, so it will be placed in the center: O C Br Br 2. Step 2: Count up the valence shell electrons using the A-group numbers fore each atom: Atom Group Valence electrons C 4A +4 e’s O 6A +6 e’s Br 7A +7 e’s Br 7A +7 e’s Net Charge 0 +0 e’s Total Valence e’s +24 e’s 1 3. Step 3: Connect the atoms with single bonds, and subtract a pair of valences electrons from the total for each bond: O C Br Br a. 24 e’s - 3(2 e’s) = 18 e’s remaining. -

Introduction 1) Lewis Structures 2) Representing Organic Structures 3) Geometry and Hybridization 4) Electronegativities And
Introduction 1) Lewis Structures 2) Representing Organic Structures 3) Geometry and Hybridization 4) Electronegativities and Dipoles 5) Resonance Structures (a) Drawing Them (b) Rules for Resonance 6) Aromaticity (a) Carbocycles (b) Heterocycles (c) Antiaromaticity 7) Tautomers and Equilibrium 8) Acidity and Basicity 9) Nucleophiles and Electrophiles (a) Nucleophilicity (b) Substrate (c) Solvent 1 Reaction mechanisms offer us insights into how reactions work / why molecules react with one another. This understanding allows us to manipulate reactions for our benefit – higher yield, control or change stereochemistry, predict new chemistry, develop new reactions and reagents, etc. To write correct, detailed mechanisms, we must: a) have a detailed knowledge of molecular structure b) represent these structures unambiguously. This chapter reviews some fundamental principles of organic chemistry. It is vitally important that we comprehend the electron distribution in a given molecule, since mechanism is the detailed description of electron movement during a reaction process. The 1st two chapters are the basic tools for proposing and writing clear and correct mechanisms. 2 (1) Lewis Structures Lewis structures are fundamental to writing and comprehending organic mechanisms. There are 2 types of Lewis structure: a) Line = covalent bond = 2 electrons b) Dot = Electron Lone pairs are often the crucial reacting parts of the molecule, so knowing if they exist or not is important. Valence electrons can be obtained from the Periodic Table. (Don’t forget that charges add or remove electrons!) Atoms strive for a full outer shell So H likes to have 2 electrons. C likes to be surrounded by 8 electrons, etc. Watch out for 3rd row elements (especially Si, P, S) that can have more than 8 electrons around them. -

Nitrogen Versus Phosphorus
The Free Atom Atomic energy levels, valence orbital ionization energies (VOIE) Electronegativity for carbon: 2.5 Electronegativity for hydrogen: 2.2 Inorganic Chemistry 5.03 The Free Atom Atomic energy levels, valence orbital ionization energies (VOIE) Electronegativity for carbon: 2.5 Electronegativity for hydrogen: 2.2 Inorganic Chemistry 5.03 The Free Atom Atomic energy levels, valence orbital ionization energies (VOIE) Electronegativity for carbon: 2.5 Electronegativity for hydrogen: 2.2 Inorganic Chemistry 5.03 The Free Atom Atomic energy levels, valence orbital ionization energies (VOIE) Quartet ground state, spin multiplicity given by 3 2S + 1 = 2( 2 ) + 1 = 4 3 1 1 3 Four possible values for the spin: + 2 , + 2 , − 2 , − 2 Inorganic Chemistry 5.03 The Free Atom Atomic energy levels, valence orbital ionization energies (VOIE) Quartet ground state, spin multiplicity given by 3 2S + 1 = 2( 2 ) + 1 = 4 3 1 1 3 Four possible values for the spin: + 2 , + 2 , − 2 , − 2 Inorganic Chemistry 5.03 The Free Atom Atomic energy levels, valence orbital ionization energies (VOIE) Quartet ground state, spin multiplicity given by 3 2S + 1 = 2( 2 ) + 1 = 4 3 1 1 3 Four possible values for the spin: + 2 , + 2 , − 2 , − 2 Inorganic Chemistry 5.03 Single versus Triple Bonds Atomic energy levels, valence orbital ionization energies (VOIE) ◦ ∆Hf for P2 is +144 kJ/mol ◦ ∆Hf for P≡N is +104 kJ/mol Inorganic Chemistry 5.03 Single versus Triple Bonds Atomic energy levels, valence orbital ionization energies (VOIE) ◦ ∆Hf for P2 is +144 kJ/mol ◦ ∆Hf for P≡N