Silanes As Ligands in Transition Metal Coordination Chemistry
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

No Rabbit Ears on Water. the Structure of the Water Molecule
No Rabbit Ears on Water The Structure of the Water Molecule: What Should We Tell the Students? Michael Laing University of Natal. Durban, South Africa In the vast majority of high school (I)and freshman uni- He also considers the possibility of s character in the bond- versity (2) chemistry textbooks, water is presented as a bent ing orbitals of OF2 and OH2 to improve the "strength" of the molecule whose bonding can be described bv the Gilles~ie- orbital (p 111) from 1.732 for purep to 2.00 for the ideal sp3. Nyholm approach with the oxygen atomsp3 Gyhridized. The Inclusion of s character in the hybrid will be "resisted in the lone uairs are said to he dominant causine the H-O-H anele case of atoms with an nnshared pair.. in the s orbital, to beieduced from the ideal 1091120to 104' and are regul&ly which is more stable than the p orbitals" (p 120). Estimates shown as equivalent in shape and size even being described of the H-E-H angle range from 91.2 for AsH3 to 93.5O for as "rabbit ears" (3). Chemists have even been advised to Hz0. He once again ascribes the anomalies for Hz0 to "re- wear Mickey Mouse ears when teaching the structure and uulsion of the charzes of the hvdroaeu atoms" (D 122). bonding of the H20molecule, presumably to emphasize the - In his famous hook (6)~itac~oro&kii describks the bond- shape of the molecule (and possibly the two equivalent lone ing in water and HzS (p 10). -

61Ni Synchrotron-Radiation-Based Mössbauer Absorption
Hyperfine Interact (2018) 239:11 https://doi.org/10.1007/s10751-018-1488-0 61Ni synchrotron-radiation-based Mossbauer¨ absorption spectroscopy of Ni nanoparticle composites Ryo Masuda1 · Hirokazu Kobayashi2,3 · Yoshimasa Aoyama2 · Makina Saito1 · Shinji Kitao1 · Hiroki Ishibashi1 · Shuichi Hosokawa1 · Takaya Mitsui4 · Yoshitaka Yoda5 · Hiroshi Kitagawa2 · Makoto Seto1,4 © Springer International Publishing AG, part of Springer Nature 2018 Abstract We obtained energy-domain 61Ni synchrotron-radiation-based Mossbauer¨ absorption spectra of three materials that relate to nanoparticles: Ni2(C8O6H2) metal- organic frameworks (MOFs), Ni nanoparticles synthesized by complete heat decomposition of the MOFs, and the composites of Ni nanoparticles and the MOFs synthesized by par- tial decomposition of the MOFs. The 61Ni abundance of all the samples was not enriched but we were successfully able to obtain their spectra in 1 day or less, by using a highly efficient measurement system where the internal conversion electrons from energy standard 61 Ni86V14 foil were detected. Although both nanoparticle constituent and MOF constituent in the composites included Ni atoms, the Mossbauer¨ parameters of the Ni nanoparticle con- stituent could be evaluated; the magnetic hyperfine field of the Ni nanoparticle constituent in the composites was different from that of the Ni nanoparticles obtained by the complete heat decomposition. This difference implied that the 3d and/or 4s electron configuration of the nanoparticle constituent were affected by the MOF constituent -

Theoretical Study of the Bis-Silylation Reaction of Ethylene Catalyzed by Titanium Dichloride Yuri Alexeev Iowa State University
Chemistry Publications Chemistry 8-2003 Theoretical Study of the Bis-Silylation Reaction of Ethylene Catalyzed by Titanium Dichloride Yuri Alexeev Iowa State University Mark S. Gordon Iowa State University, [email protected] Follow this and additional works at: http://lib.dr.iastate.edu/chem_pubs Part of the Chemistry Commons The ompc lete bibliographic information for this item can be found at http://lib.dr.iastate.edu/ chem_pubs/419. For information on how to cite this item, please visit http://lib.dr.iastate.edu/ howtocite.html. This Article is brought to you for free and open access by the Chemistry at Iowa State University Digital Repository. It has been accepted for inclusion in Chemistry Publications by an authorized administrator of Iowa State University Digital Repository. For more information, please contact [email protected]. Theoretical Study of the Bis-Silylation Reaction of Ethylene Catalyzed by Titanium Dichloride Abstract Titanium dichloride was investigated as a potential catalyst for the bis-silylation reaction of ethylene with hexachlorodisilane. Ab initio electronic structure calculations at the restricted Hartree−Fock (RHF), density functional (DFT), second-order perturbation (MP2), and couple cluster (CCSD) levels of theory were used to find optimized structures, saddle points, and minimum-energy paths that connect them. The er action was found to have a net zero barrier at the DFT, MP2, and CCSD levels of theory. Dynamic correlation is found to be important for this reaction. Disciplines Chemistry Comments Reprinted (adapted) with permission from Organometallics 22 (2003): 4111, doi:10.1021/om0303350. Copyright 2014 American Chemical Society. This article is available at Iowa State University Digital Repository: http://lib.dr.iastate.edu/chem_pubs/419 Organometallics 2003, 22, 4111-4117 4111 Theoretical Study of the Bis-Silylation Reaction of Ethylene Catalyzed by Titanium Dichloride Yuri Alexeev and Mark S. -

And Tipt-Based High-Temperature Shape Memory Alloys: a Review on Recent Advances
metals Review TiPd- and TiPt-Based High-Temperature Shape Memory Alloys: A Review on Recent Advances Yoko Yamabe-Mitarai 1,2 1 Department of Advanced Materials Science, Graduate School of Frontier Sciences, The University of Tokyo, 5-1-5 Kashiwanoha, Kashiwa-shi, Chiba 277-8561, Japan; [email protected] 2 National Institute for Materials Science, 1-2-1 Sengen, Tsukuba, Ibaraki 305-0047, Japan Received: 30 September 2020; Accepted: 12 November 2020; Published: 18 November 2020 Abstract: In this paper high-temperature shape memory alloys based on TiPd and TiPt are reviewed. The effect of the alloying elements in ternary TiPd and TiPt alloys on phase transformation and strain recovery is also discussed. Generally, the addition of alloying elements decreases the martensitic transformation temperature and improves the strength of the martensite and austenite phases. Additionally, it also decreases irrecoverable strain, but without perfect recovery due to plastic deformation. With the aim to improve the strength of high-temperature shape memory alloys, multi-component alloys, including medium- and high-entropy alloys, have been investigated and proposed as new structural materials. Notably, it was discovered that the martensitic transformation temperature could be controlled through a combination of the constituent elements and alloys with high austenite finish temperatures above 500 ◦C. The irrecoverable strain decreased in the multi-component alloys compared with the ternary alloys. The repeated thermal cyclic test was effective toward obtaining perfect strain recoveries in multi-component alloys, which could be good candidates for high-temperature shape memory alloys. Keywords: high-temperature shape memory alloys; titanium palladium; titanium platinum; multi-component alloys; medium-entropy alloys; high-entropy alloys 1. -

Zerohack Zer0pwn Youranonnews Yevgeniy Anikin Yes Men
Zerohack Zer0Pwn YourAnonNews Yevgeniy Anikin Yes Men YamaTough Xtreme x-Leader xenu xen0nymous www.oem.com.mx www.nytimes.com/pages/world/asia/index.html www.informador.com.mx www.futuregov.asia www.cronica.com.mx www.asiapacificsecuritymagazine.com Worm Wolfy Withdrawal* WillyFoReal Wikileaks IRC 88.80.16.13/9999 IRC Channel WikiLeaks WiiSpellWhy whitekidney Wells Fargo weed WallRoad w0rmware Vulnerability Vladislav Khorokhorin Visa Inc. Virus Virgin Islands "Viewpointe Archive Services, LLC" Versability Verizon Venezuela Vegas Vatican City USB US Trust US Bankcorp Uruguay Uran0n unusedcrayon United Kingdom UnicormCr3w unfittoprint unelected.org UndisclosedAnon Ukraine UGNazi ua_musti_1905 U.S. Bankcorp TYLER Turkey trosec113 Trojan Horse Trojan Trivette TriCk Tribalzer0 Transnistria transaction Traitor traffic court Tradecraft Trade Secrets "Total System Services, Inc." Topiary Top Secret Tom Stracener TibitXimer Thumb Drive Thomson Reuters TheWikiBoat thepeoplescause the_infecti0n The Unknowns The UnderTaker The Syrian electronic army The Jokerhack Thailand ThaCosmo th3j35t3r testeux1 TEST Telecomix TehWongZ Teddy Bigglesworth TeaMp0isoN TeamHav0k Team Ghost Shell Team Digi7al tdl4 taxes TARP tango down Tampa Tammy Shapiro Taiwan Tabu T0x1c t0wN T.A.R.P. Syrian Electronic Army syndiv Symantec Corporation Switzerland Swingers Club SWIFT Sweden Swan SwaggSec Swagg Security "SunGard Data Systems, Inc." Stuxnet Stringer Streamroller Stole* Sterlok SteelAnne st0rm SQLi Spyware Spying Spydevilz Spy Camera Sposed Spook Spoofing Splendide -
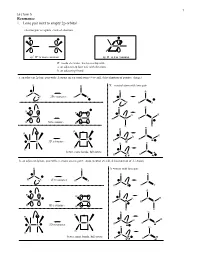
Resonance 1. Lone Pair Next to Empty 2P Orbital
1 Lecture 5 Resonance 1. Lone pair next to empty 2p orbital electron pair acceptors - lack of electrons C C CC sp2 R+ is more common sp R+ is less common R+ needs electrons, has to overlap with a. an adjacent 2p lone pair with electrons b. an adjacent pi bond a. an adjacent 2p lone pair with electrons on a neutral atom (+ overall, delocalization of positive charge) R R X = neutral atom with lone pair R R C C R X 2D resonance R X C C R X R X R R R R C R C R CX CX R N R N R 3D resonance R R R R R R R C R C R CX R O CX 3D resonance R O R R R R C better, more bonds, full octets C R F R F b. an adjacent 2p lone pair with electrons on a negative atom (neutral overall, delocalization of electrons) R R X =anion with lone pair R R C C R X 2D resonance R X C C R X R X R R R R C R C R CX CX R C R C R 3D resonance R R R R R R R C R C R CX R N CX 3D resonance R N R R R R C C better, more bonds, full octets R O R O 2 Lecture 5 Problem 1 – All of the following examples demonstrate delocalization of a lone pair of electrons into an empty 2p orbital. Usually in organic chemistry this is a carbocation site, but not always. -

Effect of Lone Pairs on Hybridization
EFFECT OF LONE PAIRS ON HYBRIDIZATION We have discussed the effect of ghost lone pairs on the shape of a molecule in the post about VSEPR theory. The effect lone pairs exert is the same in VBT as it was in VSEPR. In this post we will study a few examples in which central atom possess lone pairs. First, we will take the example of NH3 molecule. When you draw its Lewis dot structure, you will find three bonding pairs of electrons and one lone pair on N. Now write the ground state configuration of N. 7N: 1s2, 2s2, 2p3 N already has 3 unpaired electrons in ground state to make bond with 3 H atoms. You may think why does N need hybridization in this case when it has 3 unpaired electrons of the same orbital p? Because hybridized orbitals are more competent in overlapping which results in a stronger bond. 3 In NH3 molecule, N chooses sp hybridization. This may get you puzzled again, if N has 3 unpaired electrons in p why does it include s orbital which has paired electrons? You know that the atoms follow Hund’s rule for filling electrons in orbitals, similarly they have to consider energy order of orbitals in hybridization also. When an atom undergoes hybridization, it has to pick orbitals in the increasing order of their energies. In NH3, N has to first pick 2s then 2px, 2py and then 2pz. N can’t ignore 2s even if it is filled because 2s has the lowest energy and N has to include all three 2p orbitals because it needs them for bonding with three H atoms. -

How Do We Know That There Are Atoms
Chemistry 11 Fall 2010 Discussion – 12/13/10 MOs and Hybridization 1. a. [:C N:]- Bond order = (8 – 2) / 2 = 3 *2p * 2p C 2p N 2p 2p 2p *2s C 2s N 2s 2s CN- b. Comparison of Lewis and MO models of CN- CN- Consistent: Both have triple bond (B.O. = 3) Inconsistent: - Lewis structure shows lone pairs on each atom rather than all e- being shared (MO) - Lewis structure indicates a negative formal charge on C, whereas MO suggests more electron density on N, which is consistent with EN considerations c. Both molecules exhibit sp mixing, and the MOs should reflect this. In N2, the molecular orbitals would be distributed equally between the two N atoms, whereas in CN-, the bonding orbitals have greater electron density on the more electronegative atom (N). The antibonding orbitals have more electron density on the less electronegative atom (C). - 1 - Chemistry 11 Fall 2010 2. a. Completed structure of guanine (carbons at each intersection are implied): * * * * b. 17 sigma bonds; 4 pi bonds. c. All carbons are sp2 hybridized; trigonal planar; with 120˚ bond angles. d. We predict that the three N’s marked with * are sp3 hybridized, with 109.5˚ bond angles. The remaining N atoms are sp2 hybridized, with 120˚ bond angles. e. i. Benzene: ii. For the structure drawn in (a), the bond angles in the six membered ring would be expected to be 120˚ for each atom except for the N§, which is predicted to have bond angles of 109.5˚. However, it is not possible to form a planar six-membered ring unless ALL the angles are 120˚. -

Localized Electron Model for Bonding What Does Hybridization Mean?
190 Localized electron model for bonding 1. Determine the Lewis Structure for the molecule 2. Determine resonance structures 3. Determine the shape of the molecule 4. Determine the hybridization of the atoms What does hybridization mean? Why hybridize? Look at the shape of many molecules, and compare this to the shape of the orbitals of each of the atoms. Shape of orbitals Orbitals on atoms are s, p, d, and f, and there are certain shapes associated with each orbital. an s orbital is a big sphere the f orbitals look like two four leaf the p orbitals looks like “8” ’s clovers each one points in along an axis three f orbitals look like weird “8”’s with two doughnuts around each four of the d orbitals look like four one leaf clovers one d orbital looks like an “8” with a doughnut around the middle Shape of molecules Remember we determine the shape of molecules by determining the number of sets of electrons around the atom. count double and triple bonds as one set, count single bonds as one set of electrons and count lone pairs as a set 2 sets—each pair of electrons points 180° away from the other 5 sets—each pair points toward the corner of a trigonal bipyramid 3 sets—each pair points toward the corner of a triangle 6 sets—each pair points toward the corners of an octahedron 4 sets—each pair points toward the corner of a tetrahedron 191 To make bonds we need orbitals—the electrons need to go some where. -

Origin of Chalcogen-Bonding Interactions
Edinburgh Research Explorer The Origin of Chalcogen-Bonding Interactions Citation for published version: Pascoe, D, Ling, K & Cockroft, S 2017, 'The Origin of Chalcogen-Bonding Interactions', Journal of the American Chemical Society, vol. 139, no. 42, pp. 15160–15167. https://doi.org/10.1021/jacs.7b08511 Digital Object Identifier (DOI): 10.1021/jacs.7b08511 Link: Link to publication record in Edinburgh Research Explorer Document Version: Peer reviewed version Published In: Journal of the American Chemical Society General rights Copyright for the publications made accessible via the Edinburgh Research Explorer is retained by the author(s) and / or other copyright owners and it is a condition of accessing these publications that users recognise and abide by the legal requirements associated with these rights. Take down policy The University of Edinburgh has made every reasonable effort to ensure that Edinburgh Research Explorer content complies with UK legislation. If you believe that the public display of this file breaches copyright please contact [email protected] providing details, and we will remove access to the work immediately and investigate your claim. Download date: 11. Oct. 2021 The Origin of Chalcogen-Bonding Interactions Dominic J. Pascoe†, Kenneth B. Ling‡, and Scott L. Cockroft†* †EaStCHEM School of Chemistry, University of Edinburgh, Joseph Black Building, David Brewster Road, Edinburgh, EH9 3FJ, UK ‡Syngenta, Jealott’s Hill International Research Centre, Bracknell, Berkshire, RG42 6EY, UK ABSTRACT: Favorable molecular interactions between group 16 elements have been implicated in catalysis, biological processes, materials and medicinal chemistry. Such interactions have since become known as chalcogen bonds by analogy to hydrogen and halogen bonds. -

Low Pressure Chemical Vapor Deposition of A-Si:H from Disilane Cole Petersburg Iowa State University
Iowa State University Capstones, Theses and Retrospective Theses and Dissertations Dissertations 2007 Low pressure chemical vapor deposition of a-Si:H from disilane Cole Petersburg Iowa State University Follow this and additional works at: https://lib.dr.iastate.edu/rtd Part of the Electrical and Electronics Commons, and the Materials Science and Engineering Commons Recommended Citation Petersburg, Cole, "Low pressure chemical vapor deposition of a-Si:H from disilane" (2007). Retrospective Theses and Dissertations. 14557. https://lib.dr.iastate.edu/rtd/14557 This Thesis is brought to you for free and open access by the Iowa State University Capstones, Theses and Dissertations at Iowa State University Digital Repository. It has been accepted for inclusion in Retrospective Theses and Dissertations by an authorized administrator of Iowa State University Digital Repository. For more information, please contact [email protected]. Low pressure chemical vapor deposition of a-Si:H from disilane by Cole Petersburg A thesis submitted to the graduate faculty in partial fulfillment of the requirements for the degree of MASTER OF SCIENCE Major: Electrical Engineering Program of Study Committee: Vikram Dalal, Major Professor Kristen Constant Santosh Pandey Iowa State University Ames, Iowa 2007 Copyright c Cole Petersburg, 2007. All rights reserved. UMI Number: 1443091 UMI Microform 1443091 Copyright 2007 by ProQuest Information and Learning Company. All rights reserved. This microform edition is protected against unauthorized copying under Title 17, United States Code. ProQuest Information and Learning Company 300 North Zeeb Road P.O. Box 1346 Ann Arbor, MI 48106-1346 ii TABLE OF CONTENTS LIST OF TABLES . iv LIST OF FIGURES . -

Primer on Spontaneous Heating and Pyrophoricity
NOT MEASUREMENT SENSITIVE DOE‐HDBK‐1081‐2014 Supersedes DOE‐HDBK‐1081‐94 DOE HANDBOOK PRIMER ON SPONTANEOUS HEATING AND PYROPHORICITY U.S. Department of Energy FSC‐6910 Washington, D.C. 20585 DISTRIBUTION STATEMENT: Approved for public release; distribution is unlimited. This document is available on the Department of Energy Technical Standards Program Web page at: http://www.hss.doe.gov/nuclearsafety/ns/techstds/ Key words: Alkali Metals , Aluminum, Arsine, Calcium, Class D Extinguishing Agents, Coal Storage, Combustible Metals, Diborane, Fire, Hafnium, Heating, Hydrazine, Hydrocarbons, Hypergolic, Hypergolic Reaction, Iron, Lithium, Magnesium, Metals, Microbial Heating, NaK, Organic, Oxidizer, Phosphine, Phosphorus, Plutonium, Potassium, Pyrophoric, Pyrophoricity, Pyrophoric Gases, Pyrophoric Reagents, Silane Specific Area, Sodium, Sodium‐Potassium, Specific Surface Area, Spontaneous, Spontaneous Combustion, Steel, Super Oxides, Thorium, Titanium, Uranium, Water Reactive Metals, Zinc, Zirconium FOREWORD The Primer on Spontaneous Heating and Pyrophoricity is approved for use by all DOE Components. It was developed to help Department of Energy (DOE) facility contractors prevent fires caused by spontaneous ignition. Spontaneously ignitable materials include those that ignite because of a slow buildup of heat (spontaneous heating) and those that ignite in air (pyrophoricity). The scientific principles of combustion and how they affect materials known to be spontaneously combustible are explained. The fire hazards of specific spontaneously heating and pyrophoric materials are discussed as well as techniques to prevent their ignition. Suitable fire extinguishing agents are included for most materials as well as safety precautions for storage and handling. The DOE Primers are fundamental handbooks on safety‐related topics of interest in the DOE Complex and are intended as an educational aid for operations and maintenance personnel and others who may have an interest in this topic.