Nitrogen Versus Phosphorus
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Review Sheet on Determining Term Symbols
Review Sheet on Determining Term Symbols Term symbols for electronic configurations are useful not only to the spectroscopist but also to the inorganic chemist interested in understanding electronic and magnetic properties of molecules. We will concentrate on the method of Douglas and McDaniel (p. 26ff); however, you may find other treatments equal or superior to the D & M method. Term symbols are a shorthand method used to describe the energy, angular momentum, and spin multiplicity of an atom in any particular state. The general form is a given as Tj where T is a capital letter corresponding to the value of L (the angular momentum quantum number) and may be assigned as S, P, D, F, G, … for |L| = 0, 1, 2, 3, 4, … respectively. The superscript “a” is called the spin multiplicity and can be evaluated as a = 2S +1 where S is the spin quantum number. The subscript “j” is the numerical value of J, a new quantum number defined as: J = l +S, which corresponds to the total orbital and spin angular momentum of the system. The term symbol 3P is read as triplet – Pee state and indicates that there are two unpaired electrons in a state with maximum orbital angular momentum, L=1. The number of microstates (N) of a system corresponds to the total number of distinct arrangements for “e” number of electrons to be placed in “n” number of possible orbital positions. N = # of microstates = n!/(e!(n-e)!) For a set of p orbitals n = 6 since there are 2 positions in each orbital. -

No Rabbit Ears on Water. the Structure of the Water Molecule
No Rabbit Ears on Water The Structure of the Water Molecule: What Should We Tell the Students? Michael Laing University of Natal. Durban, South Africa In the vast majority of high school (I)and freshman uni- He also considers the possibility of s character in the bond- versity (2) chemistry textbooks, water is presented as a bent ing orbitals of OF2 and OH2 to improve the "strength" of the molecule whose bonding can be described bv the Gilles~ie- orbital (p 111) from 1.732 for purep to 2.00 for the ideal sp3. Nyholm approach with the oxygen atomsp3 Gyhridized. The Inclusion of s character in the hybrid will be "resisted in the lone uairs are said to he dominant causine the H-O-H anele case of atoms with an nnshared pair.. in the s orbital, to beieduced from the ideal 1091120to 104' and are regul&ly which is more stable than the p orbitals" (p 120). Estimates shown as equivalent in shape and size even being described of the H-E-H angle range from 91.2 for AsH3 to 93.5O for as "rabbit ears" (3). Chemists have even been advised to Hz0. He once again ascribes the anomalies for Hz0 to "re- wear Mickey Mouse ears when teaching the structure and uulsion of the charzes of the hvdroaeu atoms" (D 122). bonding of the H20molecule, presumably to emphasize the - In his famous hook (6)~itac~oro&kii describks the bond- shape of the molecule (and possibly the two equivalent lone ing in water and HzS (p 10). -

States of Oxygen Liquid and Singlet Oxygen Photodynamic Therapy
Oxygen States of Oxygen As far as allotropes go, oxygen as an element is fairly uninteresting, with ozone (O3, closed-shell and C2v symmetric like SO2) and O2 being the stable molecular forms. Most of our attention today will be devoted to the O2 molecule that is so critically connected with life on Earth. 2 2 2 4 2 O2 has the following valence electron configuration: (1σg) (1σu) (2σg) (1πu) (1πg) . It is be- cause of the presence of only two electrons in the two π* orbitals labelled 1πg that oxygen is paramagnetic with a triplet (the spin multiplicity \triplet" is given by 2S+1; here S, the total spin quantum number, is 0.5 + 0.5 = 1). There are six possible ways to arrange the two electrons in the two degenerate π* orbitals. These different ways of arranging the electrons in the open shell are called \microstates". Further, because there are six microstates, we can say that the total degeneracy of the electronic states 2 3 − arising from (1πg) configuration must be equal to six. The ground state of O2 is labeled Σg , the left superscript 3 indicating that this is a triplet state. It is singly degenerate orbitally and triply degenerate in terms of spin multiplicity; the total degeneracy (three for the ground state) is given by the spin times the orbital degeneracy. 1 The first excited state of O2 is labeled ∆g, and this has a spin degeneracy of one and an orbital degeneracy of two for a total degeneracy of two. This state corresponds to spin pairing of the electrons in the same π* orbital. -

Photochemical and Magnetic Properties of Complex and Nuclear Chemistry
• Programme: MSc in Chemistry • Course Code: MSCHE2001C04 • Course Title: Photochemical and magnetic properties of complex and nuclear chemistry • Unit: Electronic spectra of coordination compounds • Course Coordinator: Dr. Jagannath Roy, Associate Professor, Department of Chemistry, Central University of South Bihar Note: These materials are only for classroom teaching purpose at Central University of South Bihar. All the data/figures/materials are taken from text books, e-books, research articles, wikipedia and other online resources. 1 Course Objectives: 1. To make students understand structure and propeties of inorganic compounds 2. To accuaint the students with the electronic spectroscopy of coordination compounds 3. To introduce the concepts of magnetochemistry among the students for analysing the properties of complexes. 4. To equip the students with necessary skills in photochemical reaction transition metal complexes 5. To develop knowledge among the students on nuclear and radio chemistry Learning Outcomes: After completion of the course, learners will be able to: 1. Analyze the optical/electronic spectra of coordination compounds 2. Make use of the photochemical behaviour of complexes in designing solar cells and other applications. 3. Design and perform photochemical reactions of transition metal complexes 4. Analyze the magnetic properties of complexes 5. Explain the various phenomena taking place in the nucleus 6. Understand the working of nuclear reactors 2 Lecture-1 Lecture Topic- Introduction to the Electronic Spectra of Transition Metal complexes 3 Crystal field theory (CFT) is ideal for d1 (d9) systems but tends to fail for the more common multi- electron systems. This is because of electron-electron repulsions in addition to the crystal field effects on the repulsion of the metal electrons by the ligand electrons. -

The Chemistry of Carbene-Stabilized
THE CHEMISTRY OF CARBENE-STABILIZED MAIN GROUP DIATOMIC ALLOTROPES by MARIHAM ABRAHAM (Under the Direction of Gregory H. Robinson) ABSTRACT The syntheses and molecular structures of carbene-stabilized arsenic derivatives of 1 1 i 1 1 AsCl3 (L :AsCl3 (1); L : = :C{N(2,6- Pr2C6H3)CH}2), and As2 (L :As–As:L (2)), are presented herein. The potassium graphite reduction of 1 afforded the carbene-stabilized diarsenic complex, 2. Notably, compound 2 is the first Lewis base stabilized diatomic molecule of the Group 13–15 elements, in the formal oxidation state of zero, in the fourth period or lower of the Periodic Table. Compound 2 contains one As–As σ-bond and two lone pairs of electrons on each arsenic atom. In an effort to study the chemistry of the electron-rich compound 2, it was combined with an electron-deficient Lewis acid, GaCl3. The addition of two equivalents of GaCl3 to 2 resulted in one-electron oxidation of 2 to 1 1 •+ – •+ – give [L :As As:L ] [GaCl4] (6 [GaCl4] ). Conversely, the addition of four equivalents of GaCl3 to 2 resulted in two- electron oxidation of 2 to give 1 1 2+ – 2+ – •+ [L :As=As:L ] [GaCl4 ]2 (6 [GaCl4 ]2). Strikingly, 6 represents the first arsenic radical to be structurally characterized in the solid state. The research project also explored the reactivity of carbene-stabilized disilicon, (L1:Si=Si:L1 (7)), with borane. The reaction of 7 with BH3·THF afforded two unique compounds: one containing a parent silylene (:SiH2) unit (8), and another containing a three-membered silylene ring (9). -

Photochemical Generation of Carbenes and Ketenes from Phenanthrene-Based Precursors Part I: Dimethylalkylidene Part II: Diphenylketene
Colby College Digital Commons @ Colby Honors Theses Student Research 2017 Photochemical Generation of Carbenes and Ketenes from Phenanthrene-based Precursors Part I: Dimethylalkylidene Part II: Diphenylketene Tarini S. Hardikar Student Tarini Hardikar Colby College Follow this and additional works at: https://digitalcommons.colby.edu/honorstheses Part of the Organic Chemistry Commons Colby College theses are protected by copyright. They may be viewed or downloaded from this site for the purposes of research and scholarship. Reproduction or distribution for commercial purposes is prohibited without written permission of the author. Recommended Citation Hardikar, Tarini S. and Hardikar, Tarini, "Photochemical Generation of Carbenes and Ketenes from Phenanthrene-based Precursors Part I: Dimethylalkylidene Part II: Diphenylketene" (2017). Honors Theses. Paper 948. https://digitalcommons.colby.edu/honorstheses/948 This Honors Thesis (Open Access) is brought to you for free and open access by the Student Research at Digital Commons @ Colby. It has been accepted for inclusion in Honors Theses by an authorized administrator of Digital Commons @ Colby. Photochemical Generation of Carbenes and Ketenes from Phenanthrene-based Precursors Part I: Dimethylalkylidene Part II: Diphenylketene TARINI HARDIKAR A Thesis Presented to the Department of Chemistry, Colby College, Waterville, ME In Partial Fulfillment of the Requirements for Graduation With Honors in Chemistry SUBMITTED MAY 2017 Photochemical Generation of Carbenes and Ketenes from Phenanthrene-based Precursors Part I: Dimethylalkylidene Part II: Diphenylketene TARINI HARDIKAR Approved: (Mentor: Dasan M. Thamattoor, Professor of Chemistry) (Reader: Rebecca R. Conry, Associate Professor of Chemistry) “NOW WE KNOW” - Dasan M. Thamattoor Vitae Tarini Shekhar Hardikar was born in Vadodara, Gujarat, India in 1996. She graduated from the S.N. -

Chemical Shift (Δ)
Chapter 9 ● Nuclear Magnetic Resonance Ch. 9 - 1 1. Introduction Classic methods for organic structure determination ● Boiling point ● Refractive index ● Solubility tests ● Functional group tests ● Derivative preparation ● Sodium fusion (to identify N, Cl, Br, I & S) ● Mixture melting point ● Combustion analysis ● Degradation Ch. 9 - 2 Classic methods for organic structure determination ● Require large quantities of sample and are time consuming Ch. 9 - 3 Spectroscopic methods for organic structure determination a) Mass Spectroscopy (MS) ● Molecular Mass & characteristic fragmentation pattern b) Infrared Spectroscopy (IR) ● Characteristic functional groups c) Ultraviolet Spectroscopy (UV) ● Characteristic chromophore d) Nuclear Magnetic Resonance (NMR) Ch. 9 - 4 Spectroscopic methods for organic structure determination ● Combination of these spectroscopic techniques provides a rapid, accurate and powerful tool for Identification and Structure Elucidation of organic compounds ● Rapid ● Effective in mg and microgram quantities Ch. 9 - 5 General steps for structure elucidation 1. Elemental analysis ● Empirical formula ● e.g. C2H4O 2. Mass spectroscopy ● Molecular weight ● Molecular formula ● e.g. C4H8O2, C6H12O3 … etc. ● Characteristic fragmentation pattern for certain functional groups Ch. 9 - 6 General steps for structure elucidation 3. From molecular formula ● Double bond equivalent (DBE) 4. Infrared spectroscopy (IR) ● Identify some specific functional groups ● e.g. C=O, C–O, O–H, COOH, NH2 … etc. Ch. 9 - 7 General steps for structure elucidation 5. UV ● Sometimes useful especially for conjugated systems ● e.g. dienes, aromatics, enones 6. 1H, 13C NMR and other advanced NMR techniques ● Full structure determination Ch. 9 - 8 Electromagnetic spectrum cosmic X-rays ultraviolet visible infrared micro- radio- & γ-rays wave wave λ: 0.1nm 200nm 400nm 800nm 50µm IR X-Ray UV NMR Crystallography 1Å = 10-10m 1nm = 10-9m 1µm = 10-6m Ch. -
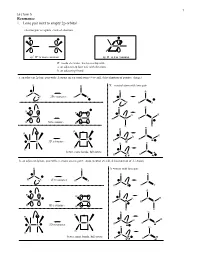
Resonance 1. Lone Pair Next to Empty 2P Orbital
1 Lecture 5 Resonance 1. Lone pair next to empty 2p orbital electron pair acceptors - lack of electrons C C CC sp2 R+ is more common sp R+ is less common R+ needs electrons, has to overlap with a. an adjacent 2p lone pair with electrons b. an adjacent pi bond a. an adjacent 2p lone pair with electrons on a neutral atom (+ overall, delocalization of positive charge) R R X = neutral atom with lone pair R R C C R X 2D resonance R X C C R X R X R R R R C R C R CX CX R N R N R 3D resonance R R R R R R R C R C R CX R O CX 3D resonance R O R R R R C better, more bonds, full octets C R F R F b. an adjacent 2p lone pair with electrons on a negative atom (neutral overall, delocalization of electrons) R R X =anion with lone pair R R C C R X 2D resonance R X C C R X R X R R R R C R C R CX CX R C R C R 3D resonance R R R R R R R C R C R CX R N CX 3D resonance R N R R R R C C better, more bonds, full octets R O R O 2 Lecture 5 Problem 1 – All of the following examples demonstrate delocalization of a lone pair of electrons into an empty 2p orbital. Usually in organic chemistry this is a carbocation site, but not always. -

Effect of Lone Pairs on Hybridization
EFFECT OF LONE PAIRS ON HYBRIDIZATION We have discussed the effect of ghost lone pairs on the shape of a molecule in the post about VSEPR theory. The effect lone pairs exert is the same in VBT as it was in VSEPR. In this post we will study a few examples in which central atom possess lone pairs. First, we will take the example of NH3 molecule. When you draw its Lewis dot structure, you will find three bonding pairs of electrons and one lone pair on N. Now write the ground state configuration of N. 7N: 1s2, 2s2, 2p3 N already has 3 unpaired electrons in ground state to make bond with 3 H atoms. You may think why does N need hybridization in this case when it has 3 unpaired electrons of the same orbital p? Because hybridized orbitals are more competent in overlapping which results in a stronger bond. 3 In NH3 molecule, N chooses sp hybridization. This may get you puzzled again, if N has 3 unpaired electrons in p why does it include s orbital which has paired electrons? You know that the atoms follow Hund’s rule for filling electrons in orbitals, similarly they have to consider energy order of orbitals in hybridization also. When an atom undergoes hybridization, it has to pick orbitals in the increasing order of their energies. In NH3, N has to first pick 2s then 2px, 2py and then 2pz. N can’t ignore 2s even if it is filled because 2s has the lowest energy and N has to include all three 2p orbitals because it needs them for bonding with three H atoms. -

How Do We Know That There Are Atoms
Chemistry 11 Fall 2010 Discussion – 12/13/10 MOs and Hybridization 1. a. [:C N:]- Bond order = (8 – 2) / 2 = 3 *2p * 2p C 2p N 2p 2p 2p *2s C 2s N 2s 2s CN- b. Comparison of Lewis and MO models of CN- CN- Consistent: Both have triple bond (B.O. = 3) Inconsistent: - Lewis structure shows lone pairs on each atom rather than all e- being shared (MO) - Lewis structure indicates a negative formal charge on C, whereas MO suggests more electron density on N, which is consistent with EN considerations c. Both molecules exhibit sp mixing, and the MOs should reflect this. In N2, the molecular orbitals would be distributed equally between the two N atoms, whereas in CN-, the bonding orbitals have greater electron density on the more electronegative atom (N). The antibonding orbitals have more electron density on the less electronegative atom (C). - 1 - Chemistry 11 Fall 2010 2. a. Completed structure of guanine (carbons at each intersection are implied): * * * * b. 17 sigma bonds; 4 pi bonds. c. All carbons are sp2 hybridized; trigonal planar; with 120˚ bond angles. d. We predict that the three N’s marked with * are sp3 hybridized, with 109.5˚ bond angles. The remaining N atoms are sp2 hybridized, with 120˚ bond angles. e. i. Benzene: ii. For the structure drawn in (a), the bond angles in the six membered ring would be expected to be 120˚ for each atom except for the N§, which is predicted to have bond angles of 109.5˚. However, it is not possible to form a planar six-membered ring unless ALL the angles are 120˚. -

Localized Electron Model for Bonding What Does Hybridization Mean?
190 Localized electron model for bonding 1. Determine the Lewis Structure for the molecule 2. Determine resonance structures 3. Determine the shape of the molecule 4. Determine the hybridization of the atoms What does hybridization mean? Why hybridize? Look at the shape of many molecules, and compare this to the shape of the orbitals of each of the atoms. Shape of orbitals Orbitals on atoms are s, p, d, and f, and there are certain shapes associated with each orbital. an s orbital is a big sphere the f orbitals look like two four leaf the p orbitals looks like “8” ’s clovers each one points in along an axis three f orbitals look like weird “8”’s with two doughnuts around each four of the d orbitals look like four one leaf clovers one d orbital looks like an “8” with a doughnut around the middle Shape of molecules Remember we determine the shape of molecules by determining the number of sets of electrons around the atom. count double and triple bonds as one set, count single bonds as one set of electrons and count lone pairs as a set 2 sets—each pair of electrons points 180° away from the other 5 sets—each pair points toward the corner of a trigonal bipyramid 3 sets—each pair points toward the corner of a triangle 6 sets—each pair points toward the corners of an octahedron 4 sets—each pair points toward the corner of a tetrahedron 191 To make bonds we need orbitals—the electrons need to go some where. -

Iaps Newsletter 2006
IAPS NEWSLETTER 2006 2006 I-APS Newsletter, Volume 28, 2006 1 Contents Letter from the President 3 Letter from the I-APS Newsletter Editor 4 I-APS Officers ` 5 2007 I-APS Awards 6 2006 Porter Awards 6 Howard Zimmerman, the 2006 Porter Medal Laureate 7 Hiroshi Masuhara, the 2006 Porter Medal Laureate 9 2006 I-APS Award Winners 11 Photographs from the I-APS Award Session 15 Research Highlights from the 2006 I-APS Award Winner: Dan Nocera, “Microlasers for High Gain Chemo-/ Bio- Sensing on Small Length Scales” 16 Reports from 2006 Photochemical Meetings 28 In Memoriam Don Arnold 40 George Hammond 41 Upcoming conferences 44 I-APS Membership Form 45 I-APS Newsletter, Volume 28, 2006 2 Letter from the President Cornelia Bohne I-APS President (2006-08) Department of Chemistry University of Victoria PO Box 3065, Victoria, BC Canada V8W 3V6 1-250-7217151 [email protected] http://www.foto.chem.uvic.ca/ August, 2006 Dear Colleagues, I became president of the society a few days after the 17th I-APS Winter Conference held in Salvador, Brazil in June. The meeting was a great success with more than 150 participants, which included about 60 students. The informal atmosphere, leading to vibrant scientific discussions, showed how well integrated the South and North American photochemical communities are. The participation of so many young photochemists bodes well for the future of the photosciences and of the society. During the meeting the society awards were presented to Dan Nocera (I-APS award), Mohammad A. Omary (Young Investigator Award), Ryan C.