Timmons Okstate 0664D 14791.Pdf (3.528Mb)
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

The Rcs Phosphorelay May Regulate the E. Coli Capsule Response to Sublethal Streptomycin Treatment
Journal of Experimental Microbiology and Immunology (JEMI) Copyright © April 2015, M&I UBC The Rcs Phosphorelay May Regulate the E. coli Capsule Response to Sublethal Streptomycin Treatment Iris Luo, Josh Wiebe, Yunan Liu Department of Microbiology and Immunology, University of British Columbia The Escherichia coli colanic acid capsule produced by the cps gene confers resistance in the presence of sublethal antibiotic concentrations. However, the exact mechanism of capsule gene regulation in reaction to the presence of antibiotics is unknown. The two main proposed cps regulation pathways both involve the Rcs phosphorelay system, but differ regarding dependence on RpoS for cps transcription. In this study, we explored the changes in capsule formation and antibiotic resistance in response to treatment with sublethal concentrations of streptomycin using deletion mutants of the two proposed regulation pathways. Wild type, Δ rcsB, Δ rpoS, and Δ cps. E. coli strains were incubated in sublethal concentrations of streptomycin. When examined with MIC assays, the deletion mutants showed higher levels of antibiotic resistance compared to wild type strains of E. coli. We also demonstrated that capsule production was induced under sublethal streptomycin conditions with Maneval staining. Furthermore, qPCR detection of cps transcription indicated downregulation in Δ rcsB strains and upregulation in Δ rpoS after streptomycin treatment. Together, these results suggest that E. coli streptomycin resistance is not dependent on capsule production, and that rcsB and rpoS may both play a role in cps regulation when treated with sublethal doses of streptomycin. Escherichia coli are rod shaped bacteria capable of producing a polysaccharide capsule. The capsule is important for protection from desiccation, survival during oxidative stress, bacterial virulence, and antibiotic resistance (1). -

Generate Metabolic Map Poster
Authors: Pallavi Subhraveti Ron Caspi Peter Midford Peter D Karp An online version of this diagram is available at BioCyc.org. Biosynthetic pathways are positioned in the left of the cytoplasm, degradative pathways on the right, and reactions not assigned to any pathway are in the far right of the cytoplasm. Transporters and membrane proteins are shown on the membrane. Ingrid Keseler Periplasmic (where appropriate) and extracellular reactions and proteins may also be shown. Pathways are colored according to their cellular function. Gcf_001591825Cyc: Bacillus vietnamensis NBRC 101237 Cellular Overview Connections between pathways are omitted for legibility. Anamika Kothari sn-glycerol phosphate phosphate pro phosphate phosphate phosphate thiamine molybdate D-xylose D-ribose glutathione 3-phosphate D-mannitol L-cystine L-djenkolate lanthionine α,β-trehalose phosphate phosphate [+ 3 more] α,α-trehalose predicted predicted ABC ABC FliY ThiT XylF RbsB RS10935 UgpC TreP PutP RS10200 PstB PstB RS10385 RS03335 RS20030 RS19075 transporter transporter of molybdate of phosphate α,β-trehalose 6-phosphate L-cystine D-xylose D-ribose sn-glycerol D-mannitol phosphate phosphate thiamine glutathione α α phosphate phosphate phosphate phosphate L-djenkolate 3-phosphate , -trehalose 6-phosphate pro 1-phosphate lanthionine molybdate phosphate [+ 3 more] Metabolic Regulator Amino Acid Degradation Amine and Polyamine Biosynthesis Macromolecule Modification tRNA-uridine 2-thiolation Degradation ATP biosynthesis a mature peptidoglycan a nascent β an N-terminal- -

Bacillus Subtilis
Das Phagenschock-Protein LiaH aus Bacillus subtilis Dissertation der Fakultät für Biologie der Ludwig-Maximilians-Universität München Vorgelegt von aus Leisnig München April 2012 1. Gutachter: Prof. Dr. Thorsten Mascher 2. Gutachterin: Prof. Dr. Ute Vothknecht Tag der Abgabe: 10.04.2012 Tag der mündlichen Prüfung: 31.10.2012 Eidesstattliche Versicherung und Erklärung Hiermit versichere ich an Eides statt, dass die vorliegende Dissertation von mir selbständig und ohne unerlaubte Hilfe angefertigt wurde. Zudem wurden keine anderen als die angegebenen Quellen verwendet. Außerdem versichere ich, dass die Dissertation keiner anderen Prüfungskommission vorgelegt wurde und ich mich nicht anderweitig einer Doktorprüfung ohne Erfolg unterzogen habe. München, den 10.04.2012 ____________________________ Diana Wolf „Nichts beflügelt die Wissenschaft so, wie der Schwatz mit Kollegen auf dem Flur.“ Arno Penzias (*1933), amerik. Physiker, 1978 Nobelpreis Inhaltsverzeichnis Inhaltsverzeichnis Publikationsliste IV Abkürzungsverzeichnis V Zusammenfassung VII Summary IX Kapitel I: Einleitung 1 I.1. Aufbau der bakteriellen Zellwand 1 I.2. Zellwandbiosynthese 2 I.3. Die Zellwand als Ziel von antibiotisch wirkenden Substanzen 5 I.4. Die Zellhüllstressantwort in Gram-positiven Bakterien am Beispiel B. subtilis 8 I.5. Das LiaFSR-Dreikomponentensystem in B. subtilis 11 I.5.1. Induktion des LiaFSR-3KS 12 I.5.2. Regulation und Funktion des LiaFSR-3KS 12 I.6. Die Zellhüllstressantwort in Gram-negativen Bakterien am Beispiel E. coli 14 I.7. Die Phagenschock-Antwort in E. coli 16 I.7.1. Induktion der Phagenschock-Antwort 17 I.7.2. Regulation der Phagenschock-Antwort 18 I.7.3. Rolle der Phagenschock-Antwort im Energiestoffwechsel 20 I.8. Die Phagenschock-Antwort in anderen Organismen 20 I.8.1. -

The Reduction of T7 Phage Adsorption in Escherichia Coli B23 Cells Treated with Sub-Lethal Levels of Kanamycin
Journal of Experimental Microbiology and Immunology (JEMI) Vol. 13:89-92 Copyright © April 2009, M&I UBC The Reduction of T7 Phage Adsorption in Escherichia coli B23 Cells Treated with Sub-Lethal Levels of Kanamycin Joanne Bleackley, Jesse Cooper, Monica Kaminski and Stephanie Sandilands Department of Microbiology & Immunology, UBC The production of capsular material has been implicated as a bacterial defence mechanism against certain antibiotics and against phage adsorption. The capsule acts to block the phage from binding to its receptor on the bacterial cell which effectively inhibits phage adsorption. Previously, it has been shown that exposing Escherichia coli B23 cells to sub-inhibitory levels of the antibiotic kanamycin results in an increase in extracellular polysaccharide production, possibly in the form of a capsule. In the current study, this phenomenon was investigated using T7 phage adsorption as a model to assess the presence of a capsule. Phage adsorption assays were performed on both E. coli B23 cells treated with sub-lethal levels of kanamycin for 1 hour and untreated cells. T7 phage adsorption was shown to be diminished in E. coli treated with antibiotic, as compared to those which had not been treated with antibiotic. This decrease in phage adsorption to the antibiotic treated cells suggests that the induced extracellular polysaccharide produced by these cells is part of the capsule. Thus, this work provides further insight into the likely nature of the induced-extracellular polysaccharide produced by these cells following antibiotic treatment. Bacteria have developed mechanisms to adapt to phage adsorption (17). Binding of T7 phage to LPS changes in their environment. -

The Effect of Skin Fatty Acids on Staphylococcus Aureus
Arch Microbiol (2015) 197:245–267 DOI 10.1007/s00203-014-1048-1 ORGINAL PAPER The effect of skin fatty acids on Staphylococcus aureus Yvonne Neumann · Knut Ohlsen · Stefanie Donat · Susanne Engelmann · Harald Kusch · Dirk Albrecht · Michael Cartron · Alexander Hurd · Simon J. Foster Received: 23 July 2014 / Revised: 19 September 2014 / Accepted: 6 October 2014 / Published online: 18 October 2014 © The Author(s) 2014. This article is published with open access at Springerlink.com Abstract Staphylococcus aureus is a commensal of the hlgBC) was reduced, whereas that of host defence evasion human nose and skin. Human skin fatty acids, in particular components (cap, sspAB, katA) was increased. In particular, cis-6-hexadecenoic acid (C-6-H), have high antistaphylococ- members of the SaeRS regulon had highly reduced expres- cal activity and can inhibit virulence determinant production. sion, and the use of specific mutants revealed that the effect Here, we show that sub-MIC levels of C-6-H result in induc- on toxin production is likely mediated via SaeRS. tion of increased resistance. The mechanism(s) of C-6-H activity was investigated by combined transcriptome and Keywords S. aureus · Skin fatty acid · C-6-H · proteome analyses. Proteome analysis demonstrated a pleio- Resistance tropic effect of C-6-H on virulence determinant production. In response to C-6-H, transcriptomics revealed altered expres- sion of over 500 genes, involved in many aspects of virulence Introduction and cellular physiology. The expression of toxins (hla, hlb, The Gram-positive bacterium Staphylococcus aureus is able to survive as a commensal organism in the anterior nares and Communicated by Djamel DRIDER. -

Analysis of the Interaction of the Luxr-Type Transcription Factors Bglj and Rcsb and Antagonism of H-NS Mediated Silencing in Escherichia Coli
Analysis of the interaction of the LuxR-type transcription factors BglJ and RcsB and antagonism of H-NS mediated silencing in Escherichia coli I n a u g u r a l - D i s s e r t a t i o n zur Erlangung des Doktorgrades der Mathematisch-Naturwissenschaftlichen Fakultät der Universität zu Köln vorgelegt von Raja Venkatesh Ganesan aus Tamil Nadu, Indien Köln, März 2010 Berichterstatter/in: Prof. Dr. Karin Schnetz Prof. Dr. Jürgen Dohmen Tag der letzten mündlichen Prüfung: 26 April 2010 Acknowledgements I take this opportunity to thank all the people who have helped, encouraged and inspired me during my graduation and made this thesis possible. First and foremost, I would like to express my deepest gratitude to my PhD supervisor Prof. Karin Schnetz for providing me the opportunity to work in her group and sharing her knowledge for the betterment of my life. I am very thankful for her valuable advice, support and teaching me the word “organization”. Thanks for the pushes and constructive criticism which helped me to make this happen. I would like to thank Prof. Dr. Siegfried Roth and Prof. Dr. Jürgen Dohmen for being part of my thesis committee. I thank Dr. Kevin Johnson for agreeing to be part of my thesis committee. I am deeply grateful to Dr. Sabari and Dr. Palani for their immeasurable help and support professionally and personally. I thank Dr. Jain Nayar for his wise words during tougher times. I also thank him for the lighter moments and great movie sessions. Many thanks to Dr. -

ABSTRACT BOZDAG, AHMET. Investigation of Methanol and Formaldehyde Metabolism in Bacillus Methanolicus
ABSTRACT BOZDAG, AHMET. Investigation of Methanol and Formaldehyde Metabolism in Bacillus methanolicus.(Under the direction of Prof. Michael C. Flickinger). Bacillus methanolicus is a Gram-positive aerobic methylotroph growing optimally at 50-53 °C. Wild-type strains of B. methanolicus have been reported to secrete 58 g/l of L- glutamate in fed-batch cultures. Mutants of B. methanolicus created via classical mutangenesis can secrete 37 g/l of L-lysine, at 50 °C. The genes required for methylotrophyin B. methanolicus are encoded by an endogenous plasmid, pBM19 in strain MGA3, except for hexulose phosphate synthase (hps) and phosphohexuloisomerase (phi) which are encoded on the chromosome.It is a promising candidate for industrial production of chemical intermediates or amino acids from methanol. B. methanolicus employs the ribulose monophospate (RuMP) pathway to assimilate the carbon derived from the methanol, but enzymes that dissimilate carbon are not identified, although formaldehyde and formate were identified as intermediates by 13C NMR. It is important to understand how methanol is oxidized to formaldehyde and then, to formate and carbon dioxide. This study aims to elucidate the methanol dissimilation pathway of B. methanolicus. Growth rates of B. methanolicus MGA3 were assessed on methanol, mannitol, and glucose as a substrate. B. methanolicus achieved maximum growth rate, µmax, when growing on 25 mM methanol, 0.65±0.007 h-1, and it gradually decreased to 0.231±0.004 h-1 at 2Mmethanol concentration which demonstrates substrate inhibition. The maximum growth rates (µmax) of B. methanolicus MGA3 on mannitol and glucose are 0.532±0.002 and 0.336±0.003 h-1, respectively. -

12) United States Patent (10
US007635572B2 (12) UnitedO States Patent (10) Patent No.: US 7,635,572 B2 Zhou et al. (45) Date of Patent: Dec. 22, 2009 (54) METHODS FOR CONDUCTING ASSAYS FOR 5,506,121 A 4/1996 Skerra et al. ENZYME ACTIVITY ON PROTEIN 5,510,270 A 4/1996 Fodor et al. MICROARRAYS 5,512,492 A 4/1996 Herron et al. 5,516,635 A 5/1996 Ekins et al. (75) Inventors: Fang X. Zhou, New Haven, CT (US); 5,532,128 A 7/1996 Eggers Barry Schweitzer, Cheshire, CT (US) 5,538,897 A 7/1996 Yates, III et al. s s 5,541,070 A 7/1996 Kauvar (73) Assignee: Life Technologies Corporation, .. S.E. al Carlsbad, CA (US) 5,585,069 A 12/1996 Zanzucchi et al. 5,585,639 A 12/1996 Dorsel et al. (*) Notice: Subject to any disclaimer, the term of this 5,593,838 A 1/1997 Zanzucchi et al. patent is extended or adjusted under 35 5,605,662 A 2f1997 Heller et al. U.S.C. 154(b) by 0 days. 5,620,850 A 4/1997 Bamdad et al. 5,624,711 A 4/1997 Sundberg et al. (21) Appl. No.: 10/865,431 5,627,369 A 5/1997 Vestal et al. 5,629,213 A 5/1997 Kornguth et al. (22) Filed: Jun. 9, 2004 (Continued) (65) Prior Publication Data FOREIGN PATENT DOCUMENTS US 2005/O118665 A1 Jun. 2, 2005 EP 596421 10, 1993 EP 0619321 12/1994 (51) Int. Cl. EP O664452 7, 1995 CI2O 1/50 (2006.01) EP O818467 1, 1998 (52) U.S. -
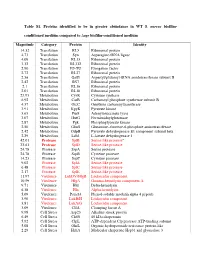
Conditioned Medium Compared to ∆Agr Biofilm-Conditioned Medium
Table S1. Proteins identified to be in greater abundance in WT S. aureus biofilm- conditioned medium compared to ∆agr biofilm-conditioned medium Magnitude Category Protein Identity 14.32 Translation RL5 Ribosomal protein 4.73 Translation Syn Asparagine-tRNA ligase 4.09 Translation RL13 Ribosomal protein 3.32 Translation RL332 Ribosomal protein 2.90 Translation EF-TU Elongation factor 2.72 Translation RL27 Ribosomal protein 2.56 Translation GatB Aspartyl/glutamyl-tRNA amidotransferase subunit B 2.42 Translation RS7 Ribosomal protein 2.1 Translation RL16 Ribosomal protein 2.01 Translation RL10 Ribosomal protein 25.93 Metabolism CysK Cysteine synthase 6.92 Metabolism CarB Carbamoyl phosphate synthetase subunit B 4.37 Metabolism OtcC Ornithine carbamoyltransferase 3.91 Metabolism KpyK Pyruvate kinase 3.63 Metabolism Pur8 Adenylosuccinate lyase 3.07 Metabolism HutG Formimidoylglutamase 2.87 Metabolism Pgk Phosphoglycerate kinase 2.50 Metabolism GlmS Glutamine--fructose-6-phosphate aminotransferase 2.42 Metabolism OdpB Pyruvate dehydrogenase E1 component subunit beta 2.29 Metabolism Ldh1 L-lactate dehydrogenase 1 42.01 Protease SplB Serine-like protease a 33.61 Protease SplD Serine-like protease 24.78 Protease SspA Serine protease 24.78 Protease SspB Cysteine protease 14.23 Protease SspP Cysteine protease 9.02 Protease SplA Serine-like protease 6.48 Protease SplC Serine-like protease 2.17 Protease SplE Serine-like protease 11.97 Virulence LukDV/HlgB Leukocidin component 10.59 Virulence HlgA Gamma-hemolysin component A 9.71 Virulence Hld Delta-hemolysin -

The Type VII Secretion System Protects Staphylococcus Aureus Against Antimicrobial Host Fatty Acids Arnaud Kengmo Tchoupa1, Kate E
www.nature.com/scientificreports OPEN The type VII secretion system protects Staphylococcus aureus against antimicrobial host fatty acids Arnaud Kengmo Tchoupa1, Kate E. Watkins1, Rebekah A. Jones1, Agnès Kuroki2, Mohammad Tauqeer Alam1, Sebastien Perrier1,2,3, Yin Chen4 & Meera Unnikrishnan1* The Staphylococcus aureus type VII secretion system (T7SS) exports several proteins that are pivotal for bacterial virulence. The mechanisms underlying T7SS-mediated staphylococcal survival during infection nevertheless remain unclear. Here we report that S. aureus lacking T7SS components are more susceptible to host-derived antimicrobial fatty acids. Unsaturated fatty acids such as linoleic acid (LA) elicited an increased inhibition of S. aureus mutants lacking T7SS efectors EsxC, EsxA and EsxB, or the membrane-bound ATPase EssC, compared to the wild-type (WT). T7SS mutants generated in diferent S. aureus strain backgrounds also displayed an increased sensitivity to LA. Analysis of bacterial membrane lipid profles revealed that the esxC mutant was less able to incorporate LA into its membrane phospholipids. Although the ability to bind labelled LA did not difer between the WT and mutant strains, LA induced more cell membrane damage in the T7SS mutants compared to the WT. Furthermore, proteomic analyses of WT and mutant cell fractions revealed that, in addition to compromising membranes, T7SS defects induce oxidative stress and hamper their response to LA challenge. Thus, our fndings indicate that T7SS contribute to maintaining S. aureus membrane integrity and homeostasis when bacteria encounter antimicrobial fatty acids. Staphylococcus aureus is a facultative pathogen that can colonize the skin and nares of healthy individuals. Te asymptomatic carriage of S. aureus is a major risk for subsequent infections 1. -

Vibrio Vulnificus: Disease and Pathogenesis (2009)
Vibrio vulnificus: Disease and Pathogenesis Melissa K. Jones and James D. Oliver Infect. Immun. 2009, 77(5):1723. DOI: 10.1128/IAI.01046-08. Published Ahead of Print 2 March 2009. Downloaded from Updated information and services can be found at: http://iai.asm.org/content/77/5/1723 These include: http://iai.asm.org/ REFERENCES This article cites 163 articles, 92 of which can be accessed free at: http://iai.asm.org/content/77/5/1723#ref-list-1 CONTENT ALERTS Receive: RSS Feeds, eTOCs, free email alerts (when new articles cite this article), more» on August 28, 2013 by Oregon State University Information about commercial reprint orders: http://journals.asm.org/site/misc/reprints.xhtml To subscribe to to another ASM Journal go to: http://journals.asm.org/site/subscriptions/ INFECTION AND IMMUNITY, May 2009, p. 1723–1733 Vol. 77, No. 5 0019-9567/09/$08.00ϩ0 doi:10.1128/IAI.01046-08 Copyright © 2009, American Society for Microbiology. All Rights Reserved. MINIREVIEW Vibrio vulnificus: Disease and Pathogenesisᰔ Melissa K. Jones and James D. Oliver* Department of Biology, University of North Carolina at Charlotte, Charlotte, North Carolina 28223 Downloaded from Vibrio vulnificus is an opportunistic human pathogen that is BIOTYPES AND GENOTYPES highly lethal and is responsible for the overwhelming majority Biotypes. Strains of V. vulnificus are classified into biotypes of reported seafood-related deaths in the United States (30, 117). This bacterium is a part of the natural flora of coastal based on their biochemical characteristics. Strains belonging to marine environments worldwide and has been isolated from biotype 1 are responsible for the majority of human infections, water, sediments, and a variety of seafood, including shrimp, while biotype 2 strains are primarily eel pathogens (2, 153). -

Viability of Engineered Biocatalysts in Biotransformation
VIABILITY OF ENGINEERED BIOCATALYSTS IN BIOTRANSFORMATION BY LOUISE DAWN HACKETT A THESIS SUBMITTED TO THE UNIVERSITY OF BIRMINGHAM FOR THE DEGREE OF MASTER OF PHILOSOPHY SCHOOL OF CHEMICAL ENGINEERING UNIVERSITY OF BIRMINGHAM NOVEMBER 2013 University of Birmingham Research Archive e-theses repository This unpublished thesis/dissertation is copyright of the author and/or third parties. The intellectual property rights of the author or third parties in respect of this work are as defined by The Copyright Designs and Patents Act 1988 or as modified by any successor legislation. Any use made of information contained in this thesis/dissertation must be in accordance with that legislation and must be properly acknowledged. Further distribution or reproduction in any format is prohibited without the permission of the copyright holder. Abstract This project aims to exploit engineered biofilms as biocatalysts in the biotransformations of enantiomerically pure compounds for fine chemical and pharmaceutical industry. It aims for conditions to be designed which would improve reactions and formation of the engineered biofilms. Tsoligkas et al. (2012) has previously engineered a biofilm to act as a biocatalyst using tryptophan synthase, TrpBA produced from plasmid pSTB7 to catalyse the biotransformation of haloindoles to L-halotryptophans. To build on this work, biofilm formation and how the cells react to the biotransformation were investigated through flow cytometry and analysis of colony forming units (CFU). For biofilms to be formed from Escherichia coli (E. Coli) K-12, it was found that the plasmid pT7-csgD had to be present or the strain required an ompR234 point mutation to allow production of curli for extracellular polymeric substances to form a biofilm.