New Advances and Challenges of Targeting Cancer Stem Cells Nurmaa K
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

Potential High-Impact Interventions Report Priority Area 02: Cancer
AHRQ Healthcare Horizon Scanning System – Potential High-Impact Interventions Report Priority Area 02: Cancer Prepared for: Agency for Healthcare Research and Quality U.S. Department of Health and Human Services 540 Gaither Road Rockville, MD 20850 www.ahrq.gov Contract No. HHSA290201000006C Prepared by: ECRI Institute 5200 Butler Pike Plymouth Meeting, PA 19462 December 2012 Statement of Funding and Purpose This report incorporates data collected during implementation of the Agency for Healthcare Research and Quality (AHRQ) Healthcare Horizon Scanning System by ECRI Institute under contract to AHRQ, Rockville, MD (Contract No. HHSA290201000006C). The findings and conclusions in this document are those of the authors, who are responsible for its content, and do not necessarily represent the views of AHRQ. No statement in this report should be construed as an official position of AHRQ or of the U.S. Department of Health and Human Services. This report’s content should not be construed as either endorsements or rejections of specific interventions. As topics are entered into the System, individual topic profiles are developed for technologies and programs that appear to be close to diffusion into practice in the United States. Those reports are sent to various experts with clinical, health systems, health administration, and/or research backgrounds for comment and opinions about potential for impact. The comments and opinions received are then considered and synthesized by ECRI Institute to identify interventions that experts deemed, through the comment process, to have potential for high impact. Please see the methods section for more details about this process. This report is produced twice annually and topics included may change depending on expert comments received on interventions issued for comment during the preceding 6 months. -

Investigator Initiated Study IRB-29839 an Open-Label Pilot Study To
Investigator Initiated Study IRB-29839 An open-label pilot study to evaluate the efficacy and safety of a combination treatment of Sonidegib and BKM120 for the treatment of advanced basal cell carcinomas Version 05 September 2016 NCT02303041 DATE: 12Dec2018 1 Coordinating Center Stanford Cancer Center 875 Blake Wilbur Drive Stanford, CA 94305 And 450 Broadway, MC 5334 Redwood City, CA 94603 Protocol Director and Principal Investigator Anne Lynn S Chang, MD, Director of Dermatological Clinical Trials 450 Broadway St, MC 5334 Redwood City, CA 94603 [email protected] Co-Investigator Anthony Oro, MD PhD 450 Broadway St, MC 5334 Redwood City, CA 94603 [email protected] Biostatistician Shufeng Li, MS 450 Broadway St, MC 5334 Redwood City, CA 94603 [email protected] Study Coordinator Ann Moffat 450 Broadway St, MC 5334 Redwood City, CA 94603 [email protected] 2 Table of Contents 1 Background ................................................................. 7 1.1 Disease Background ..................................................... 7 1.2 Hedgehog Pathway and mechanism of action ............................... 7 1.3 PI3K Pathway and mechanism of action ................................... 9 1.4 Sonidegib Compound Information ............ Error! Bookmark not defined. 1.4.1 Preclinical Studies for Sonidegib ....................................................................11 1.4.2 Muscular system...............................................................................................13 1.4.3 Skeletal system ................................................................................................13 -

Stem Cells in Cancer Initiation and Progression
REVIEW Stem cells in cancer initiation and progression Jeevisha Bajaj1,2,3,4,EmilyDiaz1,2,3,4, and Tannishtha Reya1,2,3,4 While standard therapies can lead to an initial remission of aggressive cancers, they are often only a transient solution. The resistance and relapse that follows is driven by tumor heterogeneity and therapy-resistant populations that can reinitiate Downloaded from http://rupress.org/jcb/article-pdf/219/1/e201911053/1396574/jcb_201911053.pdf by guest on 09 February 2021 growth and promote disease progression. There is thus a significant need to understand the cell types and signaling pathways that not only contribute to cancer initiation, but also those that confer resistance and drive recurrence. Here, we discuss work showing that stem cells and progenitors may preferentially serve as a cell of origin for cancers, and that cancer stem cells can be key in driving the continued growth and functional heterogeneity of established cancers. We also describe emerging evidence for the role of developmental signals in cancer initiation, propagation, and therapy resistance and discuss how targeting these pathways may be of therapeutic value. Introduction demonstrated in acute myeloid leukemia (AML; Bonnet and Cancers arise from a series of mutations or genomic alterations Dick, 1997; Lapidot et al., 1994). The identification of malignant that provide a cell with an extensive ability to evade pro- stem cells in leukemia initiated a search for similar populations apoptotic and growth-inhibitory signals and to be self-sufficient in solid tumors, and about a decade later, a small population of ingrowthsignalsthatenablethemtodivideendlessly(Nowell, cells with tumor-initiating properties were identified in mam- 1974). -

Genomic Oncology: Moving Beyond the Tip of the Iceberg Jane De Lartigue, Phd
FeatureCommunity Report Genomic oncology: moving beyond the tip of the iceberg Jane de Lartigue, PhD istorically, cancer has been diagnosed and in patients with lung cancer, even the most efec- treated on the basis of the tissue of ori- tive targeted therapies can fail if used in the wrong Hgin. Te promise of personalized therapy, patient population.5,6 matched more precisely to an individual’s tumor, In recognition of this issue, the oncology feld has was ushered in with the development of molecularly developed molecular biomarkers that can predict targeted therapies, based on a greater understanding response, or lack thereof, to targeted therapy. Drugs of cancer as a genomic-driven disease. Here, we dis- are now commonly being evaluated in trials that cuss some of the evolution of genomic oncology, the select eligible patients on the basis of biomarker pos- inherent complexities and challenges, and how novel itivity, and a number of companion diagnostics have clinical trial designs are among the strategies being been codeveloped to assist in these eforts (Table 1). developed to address them and shape the future of Notable successes include the development of the personalized medicine in cancer. monoclonal antibody trastuzumab for patients with breast cancers that have human epidermal growth The evolution of genomic oncology factor receptor 2 (HER2) gene amplifcation or In the 15 years since the frst map of the human HER2 protein overexpression,7 and the small mol- genome emerged, genetics has become an inte- ecule BRAF kinase inhibitor -
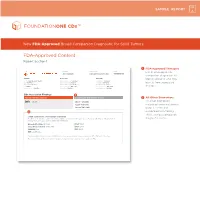
FDA-Approved Content Report Section 1
SAMPLE REPORT New FDA-Approved Broad Companion Diagnostic for Solid Tumors FDA-Approved Content Report Section 1 1 FDA-Approved Therapies PATIENT TUMOR TYPE TRF# List of FDA-approved Jane Sample Lung adenocarcinoma TRFXXXXXX companion diagnostics to PATIENT PHYSICIAN SPECIMEN identify patients who may DISEASE Lung adenocarcinoma ORDERING PHYSICIAN Not Given SPECIMEN SITE Not Given NAME Not Given MEDICAL FACILITY Not Given SPECIMEN ID Not Given benefi t from associated DATE OF BIRTH Not Given ADDITIONAL RECIPIENT Not Given SPECIMEN TYPE Not Given SEX Female MEDICAL FACILITY ID Not Given DATE OF COLLECTION Not Given therapies MEDICAL RECORD # Not Given PATHOLOGIST Not Given SPECIMEN RECEIVED Not Given CDx Associated Findings 1 GENOMIC FINDINGS DETECTED FDA-APPROVED THERAPEUTIC OPTIONS 2 All Other Biomarkers EGFR L858R Gilotrif® (Afatinib) All other biomarkers, Iressa® (Gefitinib) including tumor mutational Tarceva® (Erlotinib) burden (TMB) and 2 microsatellite instability (MSI), without companion OTHER ALTERATIONS & BIOMARKERS IDENTIFIED Results reported in this section are not prescriptive or conclusive for labeled use of any specific therapeutic product. See diagnostic claims professional services section for additional information. Microsatellite Status MS-Stable PTCH1 T416S Tumor Mutation Burden 11 Muts/Mb RBM10 Q494* CDKN2A/B loss TP53 R267P EGFR amplification § Refer to appendix for limitation statements related to detection of any copy number alterations, gene rearrangements, MSI or TMB result in this section. Please refer to appendix -

Overcoming the Challenges of Oral Oncolytic Therapies with a Specialized Crew
Navigating Safely Through Uncharted Waters: Overcoming the Challenges of Oral Oncolytic Therapies with a Specialized Crew Mitchell E. Hughes, PharmD, BCPS, BCOP Clinical Pharmacy Specialist-Hematology/Oncology The Abramson Cancer Center for Advanced Medicine at Penn Medicine Objectives At the completion of this activity, the participant will be able to: 1. List risks associated with dispensing oral oncolytic agent 2. Recognize potential barriers to implementation of a vigilance program for oral oncolytic agents 3. Discuss strategies to improve safety and communications involved with dispensing oral oncolytic agents 2 Disclosure “I have not received any commercial or financial support for this program” 3 Oral Chemotherapy Definition “Any drug you take by mouth to treat cancer. Oral chemo is not given to you with a needle. It’s a liquid or pill that you swallow.” “Chemo you swallow is as strong as other forms of chemo and works just as well. You take oral chemo at home” “But oral chemo drugs cost a lot” –The American Cancer Society 4 Image available from: https://localtvwiti.files.wordpress.com/2014/03/chemo-pills.jpg?quality=85&strip=all https://www.cancer.org/treatment/treatments-and-side-effects/treatment-types/chemotherapy/oral-chemotherapy.html Misconceptions Oral chemotherapy is less toxic than intravenous (IV) chemotherapy Oral chemotherapy requires less monitoring than IV Patients will be able to start therapy the day oral chemotherapy is prescribed Oral chemotherapy does not involve any hazardous precautions 5 Image available -

An Investigator-Initiated Open-Label Trial of Sonidegib in Advanced Basal Cell Carcinoma Patients Resistant to Vismodegib Christina Danial, Kavita Y
Published OnlineFirst November 6, 2015; DOI: 10.1158/1078-0432.CCR-15-1588 Clinical Trial Brief Report Clinical Cancer Research An Investigator-Initiated Open-Label Trial of Sonidegib in Advanced Basal Cell Carcinoma Patients Resistant to Vismodegib Christina Danial, Kavita Y. Sarin, Anthony E. Oro, and Anne Lynn S. Chang Abstract Purpose: To assess the tumor response to the smoothened sive disease with sonidegib. Three patients experienced stable (SMO) inhibitor, sonidegib (LDE225), in patients with an disease and discontinued sonidegib either due to adverse events advanced basal cell carcinoma (BCC) resistant to treatment with (n ¼ 1) or due to election for surgery (n ¼ 2). The response of one vismodegib (GDC0449). patient was not evaluable. SMO mutations with in vitro data Experimental Design: Nine patients with an advanced suggesting resistance to Hh pathway inhibition were identified BCC that was previously resistant to treatment with vismode- in 5 patients, and none of these patients experienced responses gib were given sonidegib in this investigational, open- while on sonidegib. label study. Tumor response was determined using the Conclusion: Patients with advanced BCCs that were response evaluation criteria in solid tumors. SMO mutations previously resistant to treatment with vismodegib similarly were identified using biopsy samples from the target BCC demonstrated treatment resistance with sonidegib. Patients location. who have developed treatment resistance to an SMO inhibitor Results: The median duration of treatment with sonidegib was may continue to experience tumor progression in response to 6 weeks (range, 3–58 weeks). Five patients experienced progres- other SMO inhibitors. Clin Cancer Res; 1–5. Ó2015 AACR. Introduction Sonidegib (LDE225) is a new SMO inhibitor approved in 2015 by the FDA for locally advanced BCCs. -

Combination of Ponatinib with Hedgehog Antagonist Vismodegib for Therapy-Resistant BCR-ABL1–Positive Leukemia
Published OnlineFirst January 14, 2013; DOI: 10.1158/1078-0432.CCR-12-1777 Clinical Cancer Cancer Therapy: Preclinical Research See related commentary by Dao and Tyner, p. 1309 Combination of Ponatinib with Hedgehog Antagonist Vismodegib for Therapy-Resistant BCR-ABL1–Positive Leukemia Seiichiro Katagiri1, Tetsuzo Tauchi1, Seiichi Okabe1, Yosuke Minami2, Shinya Kimura3, Taira Maekawa4, Tomoki Naoe2, and Kazuma Ohyashiki1 Abstract Purpose: The Hedgehog signaling pathway is a key regulator of cell growth and differentiation during development. Whereas the Hedgehog pathway is inactive in most normal adult tissues, Hedgehog pathway reactivation has been implicated in the pathogenesis of several neoplasms including BCR-ABL1–positive leukemia. The clear link between the Hedgehog pathway and BCR-ABL1–positive leukemia led to an effort to identify small molecules to block the pathway. Experimental Design: We investigated the combined effects of vismodegib and ponatinib, a pan-ABL1 kinase inhibitor, in nonobese diabetic/severe-combined immunodeficiency (NOD/SCID) repopulating T315I BCR-ABL1–positive cells in vitro and in vivo. Results: We observed that combination with vismodegib and ponatinib helps to eliminate therapy- resistant NOD/SCID repopulating T315I BCR-ABL1–positive cells. The percentage of CD19-positive leukemia cells in peripheral blood was significantly lower in vismodegib þ ponatinib–treated mice than that of the vehicle or ponatinib alone (P < 0.001). Spleen weights were also lower in vismodegib þ ponatinib–treated mice than in ponatinib alone (P < 0.05). Overall tumor burden, as assessed by BCR-ABL mRNA from bone marrow cells, was significantly lower in vismodegib þ ponatinib–treated mice than in ponatinib alone (P < 0.005). -

Imatinib (Gleevec™)
Biologicals What Are They? When Did All of this Happen? There are Clear Benefits. Are there also Risks? Brian J Ward Research Institute of the McGill University Health Centre Global Health, Immunity & Infectious Diseases Grand Rounds – March 2016 Biologicals Biological therapy involves the use of living organisms, substances derived from living organisms, or laboratory-produced versions of such substances to treat disease. National Cancer Institute (USA) What Effects Do Steroids Have on Immune Responses? This is your immune system on high dose steroids projects.accessatlanta.com • Suppress innate and adaptive responses • Shut down inflammatory responses in progress • Effects on neutrophils, macrophages & lymphocytes • Few problems because use typically short-term Virtually Every Cell and Pathway in Immune System ‘Target-able’ (Influenza Vaccination) Reed SG et al. Nature Medicine 2013 Nakaya HI et al. Nature Immunology 2011 Landscape - 2013 Antisense (30) Cell therapy (69) Gene Therapy (46) Monoclonal Antibodies (308) Recombinant Proteins (93) Vaccines (250) Other (81) http://www.phrma.org/sites/default/files/pdf/biologicsoverview2013.pdf Therapeutic Category Drugs versus Biologics Patented Ibuprofen (Advil™) Generic Ibuprofen BioSimilars/BioSuperiors ? www.drugbank.ca Patented Etanercept (Enbrel™) BioSimilar Etanercept Etacept™ (India) Biologics in Cancer Therapy Therapeutic Categories • Hormonal Therapy • Monoclonal antibodies • Cytokine therapy • Classical vaccine strategies • Adoptive T-cell or dendritic cells transfer • Oncolytic -

Conference Program
Conference Program Note: All Scientific Sessions will be held in the Omni Hotel at 675 L Street. Poster Sessions will be held at the Hard Rock Hotel at the corner of 5th Avenue & L Street. Sunday, January 20 7:00 p.m.-7:10 p.m. Welcoming Remarks 7:10 p.m.-8:00 p.m. Keynote Address Omni Hotel San Diego, Grand Ballroom Metastasis and diversity in breast cancer Kornelia Polyak, Dana-Farber Cancer Institute, Boston, MA 8:00 p.m.-9:30 p.m. Opening Reception Monday, January 21 7:00 a.m.-8:00 a.m. Continental Breakfast Omni Hotel San Diego, Art Gallery 8:00 a.m.-10:00 a.m. Session 1: The Soil Session Chairperson: Zena Werb, Helen Diller Family Comprehensive Cancer Center, University of California, San Francisco, CA Omni Hotel San Diego, Grand Ballroom Microenvironmental control of bone metastasis Sylvain Provot, INSERM, Paris, France The role of the microenvironment protein cathelicidin LL-37 in pancreatic ductal adenocarcinoma* Christopher Heeschen, Spanish National Cancer Research Centre (CNIO), Madrid, Spain Normalizing tumor cell metabolism in breast cancer metastasis: A novel therapeutic approach Brunhilde Felding-Habermann, Scripps Research Institute, La Jolla, CA Identification of luminal breast cancers that establish a tumor supportive macroenvironment defined by proangiogenic platelets and bone marrow derived cells* Timothy Marsh, Brigham and Women’s Hospital, Boston, MA The extracellular matrix is fertile soil Richard Hynes, Massachusetts Institute of Technology, Cambridge, MA *Short talks from proffered papers Program and Proceedings • January 20-23, 2013 • San Diego, CA 7 Program 10:00 a.m.-10:30 a.m. -

Immunology Program Seminar Speakers 1994-1995 Through 2018-2019
Immunology Program Seminar Speakers 1994-1995 through 2018-2019 Speaker Host Date Institution 2018-19 Miriam Merad, MD, PhD ZOU (co-sponsor) 9/6/18 Icahn School of Medicine at Mount Sinai Anand Balasubramani, PhD ZOU (co-sponsor) 9/6/18 AAAS Lenette Lu, MD, PhD MARKOVITZ (co-sponsor) 9/12/18 Massachusettes General Hospital Michail Lionakis, MD, ScD MARKOVITZ (co-sponsor) 10/10/18 NIH/NIAID Brian Rudd, MPH, PhD LUKACS 11/7/18 Cornell University Fred Finkelman, MD HOGAN 11/28/18 Cincinnati Children’s Hospital Michael Barry, PhD Ashley Munie 12/5/18 Mayo Clinic John Varga, MD FOX 1/9/19 Northwestern University Rachael Clark, MD GUDJONSSON 1/16/19 Brigham & Women’s Hospital Irah King, PhD SEGAL 3/20/19 McGill University Wilson Wong, PhD LEI 5/1/19 Boston University Thomas Rothstein, MD, PhD MAO-DRAAYER 5/29/19 Western Michigan University 2017-18 Avery August, PhD LUKACS 9/6/17 Cornell University Javier Carrero, PhD G. MARTINEZ-COLON 9/27/17 Washington University – St. Louis Russell Jones, PhD C. CHANG 10/11/17 McGill University John Cambier, PhD GRIGOROVA 11/15/17 University of Colorado Bernard Fox, PhD LI 2/7/18 Oregon Health & Science University Tannishtha Reya, PhD O’RIORDAN 2/21/18 University of California, San Diego Richard Bucala, MD GOLDSTEIN 3/21/18 Yale University Kristin Hogquist, PhD CARTY 4/4/18 University of Minnesota 2016-17 George Dubyak, PhD KAHLENBERG 9/7/16 Case Western University Daniel Stetson, PhD O’RIORDAN 9/21/16 University of Washington Alyssa Hasty, PhD D. -

Manufacturer Patient Assistance Programs
Manufacturer Patient Assistance Programs * Provisional Bridging Programs August 2021 highlighted below in blue For medications currently not funded per the BC Cancer Benefit Drug List - http://www.bccancer.bc.ca/systemic-therapy-site/documents/policy%20and%20forms/benefit%20drug%20list.pdf Disclaimer: BC Cancer intends to keep the information on this document as up to date as possible but cannot guarantee that the programs are all available as listed. Contact your local Drug Access Navigator (DAN) for more information UPDATES: New MPAP/Compassionate Access Open: Abiraterone-JAMP, Abiraterone-Sandoz, Everolimus-Sandoz, Gefitinib-JAMP, Gefinitib-Sandoz, Imatinib-JAMP, Encorafenib (Braftovi), Binimetinib (Mektovi), Pralsetinib (Gavreto), Trastuzumab Deruxtecan (Enhertu) Provisional Bridging/Compassionate Access Closed: MPAP Closed: Additional Updates: Drug Manufacturer / PAP Name Contact Information Support Offered Route Strength DIN Abemaciclib (Verzenio) Lilly Phone 1.855.545.5922 Compassionate supply may be available PO 50mg 02487098 * Provisional Bridging Program Lilly Patient Support Program Fax 1.844.503.7749 Financial assistance for patients with or without private insurance may be available PO 100mg 02487101 Email [email protected] PO 150mg 02487128 Web PO 200mg 02487136 Abiraterone (Zytiga) Janssen Phone 1.844.511.2616 Compassionate supply may be available PO 250mg 02371065 * Provisional Bridging Program Janssen BioAdvance Patient Assistance Program Fax 1.855.629.7100 Financial assistance for patients with or without private