The Insecticide and Miticide Mode of Action Field Guide1 a Resource to Assist in Managing Arthropod Pests of Turfgrass and Ornamental Plants
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

Michigan Hop Management Guide 2018
2018 Michigan Hop Management Guide This material is based upon work supported by the National Institute of Food and Agriculture, U.S. Department of Agriculture, under Agreement No. 2015-09785. Any opinions, findings, conclusions, or recommendations expressed in this publication are those of the author(s) and do not necessarily reflect the view of the U.S. Department of Agriculture. 2 Table of Contents Growth Stages………………………………………………………………………………………3 Weed Management………………………………………………………………………….4-5 Herbicides………………..……………………………………………………………………….6-7 Fungicides……………………………..………………………………………………………….8-9 Insecticides…………………………..………………………………………………………10-11 Miticides…………………………………………………………………………………………….12 Pesticide Toxicity to Beneficial Insects…………………………………………..13-14 Nutrient Management Considerations…………………………………………15-19 Scouting Calendar………………………………………………………………………………20 Information presented here does not supersede the label directions. To protect yourself, others, and the environment, always read the label before applying any pesticide. Although efforts have been made to check the accuracy of information presented, it is the responsibility of the person using this information to verify that it is correct by reading the corresponding pesticide label in its entirety before using the product. The information presented here is intended as a guide for Michigan hop growers in selecting pesticides and is for educational purposes only. Labels can and do change. For current label and MSDS information, visit one of the following free online databases: greenbook.net, cdms.com, and agrian.com The efficacies of products listed have not been evaluated on hop in Michigan. Reference to commercial products or trade names does not imply endorsement by Michigan State University Extension or bias against those not mentioned. This information was compiled by Erin Lizotte and Dr. Robert Sirrine with assistance from Dr. -

Identifying the Cause of Sediment Toxicity in Agricultural Sediments: the Role of Pyrethroids and Nine Seldom-Measured Hydrophobic Pesticides ⇑ Donald P
Chemosphere 90 (2013) 958–964 Contents lists available at SciVerse ScienceDirect Chemosphere journal homepage: www.elsevier.com/locate/chemosphere Identifying the cause of sediment toxicity in agricultural sediments: The role of pyrethroids and nine seldom-measured hydrophobic pesticides ⇑ Donald P. Weston a, , Yuping Ding b, Minghua Zhang c, Michael J. Lydy b a Department of Integrative Biology, University of California, 1005 Valley Life Sciences Bldg., Berkeley, CA 94720-3140, USA b Fisheries and Illinois Aquaculture Center and Department of Zoology, Southern Illinois University, 171 Life Sciences II, Carbondale, IL 62901, USA c Department of Land, Air, and Water Resources, University of California, Davis, CA 95616, USA highlights " Monitoring fails to test for many agricultural pesticides used in any given area. " Nine seldom-analyzed pesticides (e.g., abamectin) were tested for in sediments. " One-quarter of the sediment samples were toxic to the amphipod, Hyalella azteca. " The seldom-analyzed pesticides may have contributed to toxicity in a few samples. " Pyrethroid insecticides were responsible for the vast majority of toxicity. article info abstract Article history: Few currently used agricultural pesticides are routinely monitored for in the environment. Even if Received 10 January 2012 concentrations are known, sediment LC50 values are often lacking for common sediment toxicity testing Received in revised form 16 May 2012 species. To help fill this data gap, sediments in California’s Central Valley were tested for nine hydropho- Accepted 27 June 2012 bic pesticides seldom analyzed: abamectin, diazinon, dicofol, fenpropathrin, indoxacarb, methyl para- Available online 23 July 2012 thion, oxyfluorfen, propargite, and pyraclostrobin. Most were detected, but rarely at concentrations acutely toxic to Hyalella azteca or Chironomus dilutus. -

Historical Perspectives on Apple Production: Fruit Tree Pest Management, Regulation and New Insecticidal Chemistries
Historical Perspectives on Apple Production: Fruit Tree Pest Management, Regulation and New Insecticidal Chemistries. Peter Jentsch Extension Associate Department of Entomology Cornell University's Hudson Valley Lab 3357 Rt. 9W; PO box 727 Highland, NY 12528 email: [email protected] Phone 845-691-7151 Mobile: 845-417-7465 http://www.nysaes.cornell.edu/ent/faculty/jentsch/ 2 Historical Perspectives on Fruit Production: Fruit Tree Pest Management, Regulation and New Chemistries. by Peter Jentsch I. Historical Use of Pesticides in Apple Production Overview of Apple Production and Pest Management Prior to 1940 Synthetic Pesticide Development and Use II. Influences Changing the Pest Management Profile in Apple Production Chemical Residues in Early Insect Management Historical Chemical Regulation Recent Regulation Developments Changing Pest Management Food Quality Protection Act of 1996 The Science Behind The Methodology Pesticide Revisions – Requirements For New Registrations III. Resistance of Insect Pests to Insecticides Resistance Pest Management Strategies IV. Reduced Risk Chemistries: New Modes of Action and the Insecticide Treadmill Fermentation Microbial Products Bt’s, Abamectins, Spinosads Juvenile Hormone Analogs Formamidines, Juvenile Hormone Analogs And Mimics Insect Growth Regulators Azadirachtin, Thiadiazine Neonicotinyls Major Reduced Risk Materials: Carboxamides, Carboxylic Acid Esters, Granulosis Viruses, Diphenyloxazolines, Insecticidal Soaps, Benzoyl Urea Growth Regulators, Tetronic Acids, Oxadiazenes , Particle Films, Phenoxypyrazoles, Pyridazinones, Spinosads, Tetrazines , Organotins, Quinolines. 3 I Historical Use of Pesticides in Apple Production Overview of Apple Production and Pest Management Prior to 1940 The apple has a rather ominous origin. Its inception is framed in the biblical text regarding the genesis of mankind. The backdrop appears to be the turbulent setting of what many scholars believe to be present day Iraq. -

Neuroactive Insecticides: Targets, Selectivity, Resistance, and Secondary Effects
EN58CH06-Casida ARI 5 December 2012 8:11 Neuroactive Insecticides: Targets, Selectivity, Resistance, and Secondary Effects John E. Casida1,∗ and Kathleen A. Durkin2 1Environmental Chemistry and Toxicology Laboratory, Department of Environmental Science, Policy, and Management, 2Molecular Graphics and Computational Facility, College of Chemistry, University of California, Berkeley, California 94720; email: [email protected], [email protected] Annu. Rev. Entomol. 2013. 58:99–117 Keywords The Annual Review of Entomology is online at acetylcholinesterase, calcium channels, GABAA receptor, nicotinic ento.annualreviews.org receptor, secondary targets, sodium channel This article’s doi: 10.1146/annurev-ento-120811-153645 Abstract Copyright c 2013 by Annual Reviews. Neuroactive insecticides are the principal means of protecting crops, people, All rights reserved livestock, and pets from pest insect attack and disease transmission. Cur- ∗ Corresponding author rently, the four major nerve targets are acetylcholinesterase for organophos- phates and methylcarbamates, the nicotinic acetylcholine receptor for neonicotinoids, the γ-aminobutyric acid receptor/chloride channel for by Public Health Information Access Project on 04/29/14. For personal use only. Annu. Rev. Entomol. 2013.58:99-117. Downloaded from www.annualreviews.org polychlorocyclohexanes and fiproles, and the voltage-gated sodium channel for pyrethroids and dichlorodiphenyltrichloroethane. Species selectivity and acquired resistance are attributable in part to structural differences in binding subsites, receptor subunit interfaces, or transmembrane regions. Additional targets are sites in the sodium channel (indoxacarb and metaflumizone), the glutamate-gated chloride channel (avermectins), the octopamine receptor (amitraz metabolite), and the calcium-activated calcium channel (diamides). Secondary toxic effects in mammals from off-target serine hydrolase inhibi- tion include organophosphate-induced delayed neuropathy and disruption of the cannabinoid system. -

Manual for Certificate Course on Plant Protection & Pesticide Management
Manual for Certificate Course on Plant Protection & Pesticide Management (for Pesticide Dealers) For Internal circulation only & has no legal validity Compiled by NIPHM Faculty Department of Agriculture , Cooperation& Farmers Welfare Ministry of Agriculture and Farmers Welfare Government of India National Institute of Plant Health Management Hyderabad-500030 TABLE OF CONTENTS Theory Practical CHAPTER Page No. class hours hours I. General Overview and Classification of Pesticides. 1. Introduction to classification based on use, 1 1 2 toxicity, chemistry 2. Insecticides 5 1 0 3. fungicides 9 1 0 4. Herbicides & Plant growth regulators 11 1 0 5. Other Pesticides (Acaricides, Nematicides & 16 1 0 rodenticides) II. Pesticide Act, Rules and Regulations 1. Introduction to Insecticide Act, 1968 and 19 1 0 Insecticide rules, 1971 2. Registration and Licensing of pesticides 23 1 0 3. Insecticide Inspector 26 2 0 4. Insecticide Analyst 30 1 4 5. Importance of packaging and labelling 35 1 0 6. Role and Responsibilities of Pesticide Dealer 37 1 0 under IA,1968 III. Pesticide Application A. Pesticide Formulation 1. Types of pesticide Formulations 39 3 8 2. Approved uses and Compatibility of pesticides 47 1 0 B. Usage Recommendation 1. Major pest and diseases of crops: identification 50 3 3 2. Principles and Strategies of Integrated Pest 80 2 1 Management & The Concept of Economic Threshold Level 3. Biological control and its Importance in Pest 93 1 2 Management C. Pesticide Application 1. Principles of Pesticide Application 117 1 0 2. Types of Sprayers and Dusters 121 1 4 3. Spray Nozzles and Their Classification 130 1 0 4. -

INDEX to PESTICIDE TYPES and FAMILIES and PART 180 TOLERANCE INFORMATION of PESTICIDE CHEMICALS in FOOD and FEED COMMODITIES
US Environmental Protection Agency Office of Pesticide Programs INDEX to PESTICIDE TYPES and FAMILIES and PART 180 TOLERANCE INFORMATION of PESTICIDE CHEMICALS in FOOD and FEED COMMODITIES Note: Pesticide tolerance information is updated in the Code of Federal Regulations on a weekly basis. EPA plans to update these indexes biannually. These indexes are current as of the date indicated in the pdf file. For the latest information on pesticide tolerances, please check the electronic Code of Federal Regulations (eCFR) at http://www.access.gpo.gov/nara/cfr/waisidx_07/40cfrv23_07.html 1 40 CFR Type Family Common name CAS Number PC code 180.163 Acaricide bridged diphenyl Dicofol (1,1-Bis(chlorophenyl)-2,2,2-trichloroethanol) 115-32-2 10501 180.198 Acaricide phosphonate Trichlorfon 52-68-6 57901 180.259 Acaricide sulfite ester Propargite 2312-35-8 97601 180.446 Acaricide tetrazine Clofentezine 74115-24-5 125501 180.448 Acaricide thiazolidine Hexythiazox 78587-05-0 128849 180.517 Acaricide phenylpyrazole Fipronil 120068-37-3 129121 180.566 Acaricide pyrazole Fenpyroximate 134098-61-6 129131 180.572 Acaricide carbazate Bifenazate 149877-41-8 586 180.593 Acaricide unclassified Etoxazole 153233-91-1 107091 180.599 Acaricide unclassified Acequinocyl 57960-19-7 6329 180.341 Acaricide, fungicide dinitrophenol Dinocap (2, 4-Dinitro-6-octylphenyl crotonate and 2,6-dinitro-4- 39300-45-3 36001 octylphenyl crotonate} 180.111 Acaricide, insecticide organophosphorus Malathion 121-75-5 57701 180.182 Acaricide, insecticide cyclodiene Endosulfan 115-29-7 79401 -

Genetically Modified Baculoviruses for Pest
INSECT CONTROL BIOLOGICAL AND SYNTHETIC AGENTS This page intentionally left blank INSECT CONTROL BIOLOGICAL AND SYNTHETIC AGENTS EDITED BY LAWRENCE I. GILBERT SARJEET S. GILL Amsterdam • Boston • Heidelberg • London • New York • Oxford Paris • San Diego • San Francisco • Singapore • Sydney • Tokyo Academic Press is an imprint of Elsevier Academic Press, 32 Jamestown Road, London, NW1 7BU, UK 30 Corporate Drive, Suite 400, Burlington, MA 01803, USA 525 B Street, Suite 1800, San Diego, CA 92101-4495, USA ª 2010 Elsevier B.V. All rights reserved The chapters first appeared in Comprehensive Molecular Insect Science, edited by Lawrence I. Gilbert, Kostas Iatrou, and Sarjeet S. Gill (Elsevier, B.V. 2005). All rights reserved. No part of this publication may be reproduced or transmitted in any form or by any means, electronic or mechanical, including photocopy, recording, or any information storage and retrieval system, without permission in writing from the publishers. Permissions may be sought directly from Elsevier’s Rights Department in Oxford, UK: phone (þ44) 1865 843830, fax (þ44) 1865 853333, e-mail [email protected]. Requests may also be completed on-line via the homepage (http://www.elsevier.com/locate/permissions). Library of Congress Cataloging-in-Publication Data Insect control : biological and synthetic agents / editors-in-chief: Lawrence I. Gilbert, Sarjeet S. Gill. – 1st ed. p. cm. Includes bibliographical references and index. ISBN 978-0-12-381449-4 (alk. paper) 1. Insect pests–Control. 2. Insecticides. I. Gilbert, Lawrence I. (Lawrence Irwin), 1929- II. Gill, Sarjeet S. SB931.I42 2010 632’.7–dc22 2010010547 A catalogue record for this book is available from the British Library ISBN 978-0-12-381449-4 Cover Images: (Top Left) Important pest insect targeted by neonicotinoid insecticides: Sweet-potato whitefly, Bemisia tabaci; (Top Right) Control (bottom) and tebufenozide intoxicated by ingestion (top) larvae of the white tussock moth, from Chapter 4; (Bottom) Mode of action of Cry1A toxins, from Addendum A7. -

2.13 Fipronil Effect on the Frequency of Anomalous Brood in Honeybee Reared in Vitro Carina A.S
Hazards of pesticides to bees - 12th International Symposium of the ICP-PR Bee Protection Group, Ghent (Belgium), September 15-17, 2014 2.13 Fipronil effect on the frequency of anomalous brood in honeybee reared in vitro Carina A.S. Silva1, Elaine C.M. Silva-Zacarin2, Caio E.C. Domingues2, Fábio C. Abdalla2, Osmar Malaspina3, Roberta C.F. Nocelli1*. 1CCA - Centro de Ciências Agrarias, UFSCar - Universidade Federal de São Carlos. Rod. Anhanguera, SP 330, Km. 174, Araras – SP, Brasil. Email: [email protected]; Email: [email protected], *Phone: +55 (19) 3543- 2595 2LABEF – Laboratório de Biologia Estrutural e Funcional. Universidade Federal de São Carlos – UFSCar. Rodovia João Leme dos Santos (SP-264), Km. 110, Bairro Itinga, Sorocaba – SP, Brasil. 3CEIS – Centro de Estudos de Insetos Sociais. Universidade Estadual “Julio de Mesquita Filho” - UNESP. Av. 24 A, 1515, Bela Vista, Rio Claro – SP, Brasil. Abstract Larvae of honeybee workers were exposed to the insecticide fipronil during the feeding phase. To evaluate the effect of fipronil in the post-embryonic development of africanized Apis mellifera, bioassays of toxicity were done. The bioassays were performed by acute exposure applying 1μL of distilled water for control (I) and for experiments: 0.5 ng a.i./µL of fipronil; 5 ng a.i./µL of fipronil and 20 ng a.i./ µL of fipronil. Triplicates were performed for all treatments. The results showed that the rate of anomalous pupae in exposed honeybees was statistically significant in relationship to the control (p <0:03). The most frequent abnormalities were: high pigmentation on the proximal and distal larval body and body malformation, such as absence of head and limbs. -

(12) United States Patent (10) Patent No.: US 8,852,618 B2 Clough (45) Date of Patent: Oct
USOO8852618B2 (12) United States Patent (10) Patent No.: US 8,852,618 B2 Clough (45) Date of Patent: Oct. 7, 2014 (54) INSECTICIDAL MIXTURE CONTAINING CA 2429218 A1 6, 2002 GAMMA-CYHALOTHRN CH 689326 A5 4f1995 EP O237227 A1 9, 1987 EP 0771526 A2 5, 1997 (75) Inventor: Martin Stephen Clough, Bracknell EP O988788 A1 3f2000 (GB) FR 272O230 A1 12/1995 JP 63. 126805 A2 5, 1988 (73) Assignee: Syngenta Limited, Guildford (GB) JP 63126805 A2 5, 1988 JP 63126805 5, 1998 c - r WO WO 86 O7525 A1 12, 1986 (*) Notice: Subject to any disclaimer, the term of this WO WO 93 03618 A2 3, 1993 patent is extended or adjusted under 35 WO WO95 229O2 A1 8/1995 U.S.C. 154(b) by 824 days. WO WO9533380 A1 12, 1995 WO WO 96 16543 A2 6, 1996 (21) Appl. No.: 12/633,063 WO WO97 06687 A1 2/1997 WO WO974O692 A1 11, 1997 (22) Filed: Dec.a V88, 2009 WO WOOOO2453 A1 1, 2000 OTHER PUBLICATIONS (65) Prior Publication Data US 201O/OO81714 A1 Apr. 1, 2010 Canadian Office Action (Applin. No. 2,452,515 filed: Jul. 10, 2002) mailing date Oct. 1, 2010 (pp. 1-2). Related U.S. Application Data Allen et al. Transgenic & Conventional Insect & Weed Control Sys tems; Proceedings of the Beltwide Cotton Conference, vol. 2, 1065 (62) Division of application No. 10/484.745, filed as 1068 (1999), USA. application No. PCT/GB02/03181 on Jul. 10, 2002, Anonymous; Pesticide Mixtures for Control of Insect and Acarid now Pat. No. -

Best Management Practices for Brown Dog Ticks
Best Management Practices For Brown Dog Ticks immediately seek shelter in cracks or crevices such as baseboards or furniture, but also commonly move en masse up walls and congregate in the corners of ceilings. These larvae eventually find the dog and receive their first blood meal. The larvae go largely unnoticed because they are about the size of a pencil tip. The larvae then drop off into the surrounding area, molt to the nymphal stage, and again seek the dog for a second blood meal. At this point owners occasionally notice the ticks, but they usually go unnoticed. After they molt to the adult stage, the ticks find the dog for a third and final blood meal. Ticks are noticed at this stage for two reasons: 1) Adult females engorge to the size of a raisin and are often located on or near the dog’s head, or 2) adults are seen Heavy infestation of BDT on the ear of a dog. crawling on floors actively looking for the dog. This “predatory” behavior is somewhat unique to ticks. The brown dog tick (BDT) can be a serious Typically, residents do not notice these ticks until they have pest in homes with pets. These Best Management completed a full generation. Often, overlapping generations of Practices are designed to support cooperation ticks occur in homes, so tick numbers can quickly multiply into the thousands. between homeowners and pest management A factor complicating BDT management is the ability of this professionals in order to prevent and control BDTs. tick to survive without a host for several months during each of At left: The black-legged its three life stages, thereby negating the “wait-it-out” strategy of tick (Ixodes scapularis). -

Validation Report 28
EURL for Cereals and Feeding stuff National Food Institute Technical University of Denmark Validation Report 28 Determination of pesticide residues in hay by LC-MS/MS and GC-MS/MS (QuEChERS method) Susan Strange Herrmann Mette Erecius Poulsen February 2018 Page 2 of 67 CONTENT: 1. Introduction ...................................................................................................................................... 3 2. Principle of analysis......................................................................................................................... 3 3. Validation design ............................................................................................................................. 4 4. Calibration curves............................................................................................................................ 4 5. Validation parameters...................................................................................................................... 4 6. Criteria for the acceptance of validation results ............................................................................. 5 7. Results and conclusion ..................................................................................................................... 6 9. References ........................................................................................................................................ 6 Appendix 1a. GCMSMS transitions used for validation of pesticides in Hay .................................... -
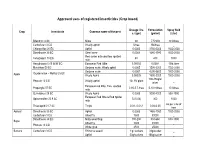
Approved Uses of Registered Insecticides (Crop Based)
Approved uses of registered insecticides (Crop based) Dosage / ha Formulation Spray fluid Crop Insecticide Common name of the pest a.i (gm) (gm/ml) (Liter) Bifenthrin 8 SC Mites 60 7.5ml/lit 10 lit/tree Carbofuran 3 CG Woolly aphid 5/tree 166/tree _ Chlorpyrifos 20 EC Aphid 0.0005 3750-5000 1500-2000 Dimethoate 30 EC Stem borer 0.0003 1485-1980 1500-2000 Red spider mite and two spotted Fenazaquin 10 EC 40 400 1000 mite Hexythiazox 5.45 W/W EC European Red Mite 0.00002 0.0004 10ltr./tree Malathion 50 EC Sanjose scale, Wooly aphid 0.0005 1500-2000 1500-2000 Sanjose scale 0.0007 4200-5600 1500-2000 Oxydemeton – Methyl 25 EC Apple Wooly Aphid 0.00025 1500-2000 1500-2000 100-150gm/ Phorate 10 CG Woolly aphid 10- 15/ plant _ plant European red Mite, Two spotted Propargite 57 EC 2.85-5.7 /tree 5-10 ml/tree 10 lit/tree mite Quinalphos 25 EC Wooly Aphid 0.0005 3000-4000 500-1000 European Red Mite & Red Spider Spiromesifen 22.9 SC 72(0.03) 300 1000 mite As per size of Thiacloprid 21.7 SC Thrips 0.01- 0.012 0.04-0.05 tree Apricot Dimethoate 30 EC Aphid 0.0003 1485-1980 1500-2000 Carbofuran 3 CG Shoot fly 1500 50000 _ Dimethoate 30 EC Milky weed bug 180-200 594-660 500-1000 Bajra Shoot fly 3000 30000 _ Phorate 10 CG White grub 2500 25000 _ Banana Carbofuran 3 CG Rhizome weevil 1 g/ suckers 33g/sucker _ Aphid 50g/suckers 166g/sucker _ Nematode 1.5g/suckers 50g/suckers _ Dimethoate 30 EC Aphid, Lace wing bug 0.0003 1485-1980 1500-2000 Tingyi bug 0.00025 1500-2000 1500-2000 Oxydemeton – Methyl 25 EC Aphids 0.0005 3000-4000 1500-2000 2.5 -1.25/ 25 -12.5/ Phorate 10 CG Aphid _ plant plant Quinalphos 25 EC Tingid bug 0.0005 3000-4000 500-1000 Phosalone 35 EC Aphid 500 1428 500-1000 Aphid 1000 33300 _ Barely Carbofuran 3 CG Jassid 1250 41600 _ Cyst nematode 1000 33300 _ Phorate 10 CG Aphid 1000 10000 _ Beans Chlorpyrifos 20 EC Pod borer , Black bug 600 3000 500-1000 Chlorantraniliprole 18.5 SC Pod borers 25 125 500 Chlorpyrifos 1.5 DP Helicoverpa armigera 375 25000 _ Azadirachtin 0.03 (300 PPM) Pod Borer _ _ _ Bacillus thuringiensis Var.