Desmidopora and Nodulipora: Misfits in the Coral World
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

Silurian Rugose Corals of the Central and Southwest Great Basin
Silurian Rugose Corals of the Central and Southwest Great Basin GEOLOGICAL SURVEY PROFESSIONAL PAPER 777 Silurian Rugose Corals of the Central and Southwest Great Basin By CHARLES W. MERRIAM GEOLOGICAL SURVEY PROFESSIONAL PAPER 777 A stratigraphic-paleontologic investigation of rugose corals as aids in age detern2ination and correlation of Silurian rocks of the Great Basin with those of other regions UNITED STATES GOVERNMENT PRINTING OFFICE, WASHINGTON 1973 UNITED STATES DEPARTMENT OF THE INTERIOR ROGERS C. B. MORTON, Secretary GEOLOGICAL SURVEY V. E. McKelvey, Director Library of Congress catalog-card No. 73-600082 For sale by the Superintendent of Documents, U.S. Government Printing Office Washington, D.C. 20402- Price $2.15 (paper cover) Stock Number 2401-00363 CONTENTS Page Page Abstract--------------------------------------------------------------------------- 1 Systematic and descriptive palaeontology-Continued Introduction -------------------------------------------------------------------- 1 Family Streptelasmatidae-Continued Purpose and scope of investigation------------------------------- 1 Dalmanophyllum ------------------------------------------------- 32 History of investigation ---------------------------------------------- 1 Family Stauriidae ------------------------------------------------------- 32 Methods of study------------------------------------------------------- 2 Cyathoph y llo ides-------------------------------------------------- 32 Acknowledgments------------------------------------------------------- 4 Palaeophyllum -

Corals (Anthozoa, Tabulata and Rugosa)
Bulletin de l’Institut Scientifique, Rabat, section Sciences de la Terre, 2008, n°30, 1-12. Corals (Anthozoa, Tabulata and Rugosa) and chaetetids (Porifera) from the Devonian of the Semara area (Morocco) at the Museo Geominero (Madrid, Spain), and their biogeographic significance Andreas MAY Saint Louis University - Madrid campus, Avenida del Valle 34, E-28003 Madrid, Spain e-mail: [email protected] Abstract. The paper describes the three tabulate coral species Caliapora robusta (Pradáčová, 1938), Pachyfavosites tumulosus Janet, 1965 and Thamnopora major (Radugin, 1938), the rugose coral Phillipsastrea ex gr. irregularis (Webster & Fenton in Fenton & Fenton, 1924) and the chaetetid Rhaphidopora crinalis (Schlüter, 1880). The specimens are described for the first time from Givetian and probably Frasnian strata of Semara area (Morocco, former Spanish Sahara). The material is stored in the Museo Geominero in Madrid. The tabulate corals and the chaetetid demonstrate close biogeographic relationships to Central and Eastern Europe as well as to Western Siberia. The fauna does not show any special influence of the Eastern Americas Realm. Key words: Anthozoa, biogeography, Devonian, tabulate corals, Morocco, West Sahara palaeogeographic province Coraux (Anthozoa, Tabulata et Rugosa) et chaetétides (Porifera) du Dévonien de la région de Smara (Maroc) déposés au Museo Geominero (Madrid) et leur signification biogéographique. Résumé. L´article décrit trois espèces de coraux tabulés : Caliapora robusta (Pradáčová, 1938), Pachyfavosites tumulosus Janet, 1965, et Thamnopora major (Radugin, 1938), le corail rugueux Phillipsastrea ex gr. irregularis (Webster & Fenton in Fenton & Fenton, 1924) ainsi que le chaetétide Rhaphidopora crinalis (Schlüter, 1880). Les spécimens, entreposés au Museo Geominero de Madrid, proviennent des couches givétiennes et probablement frasniennes de différents gisements de la région de Smara (Maroc, ancien Sahara espagnol), d’où elles sont décrites pour la première fois. -

A New Early Visean Coral Assemblage from Azrou-Khenifra Basin, Central Morocco and Palaeobiogeographic Implications Sergio Rodríguez1,2* , Ian D
Rodríguez et al. Journal of Palaeogeography (2020) 9:5 https://doi.org/10.1186/s42501-019-0051-5 Journal of Palaeogeography ORIGINAL ARTICLE Open Access A new early Visean coral assemblage from Azrou-Khenifra Basin, central Morocco and palaeobiogeographic implications Sergio Rodríguez1,2* , Ian D. Somerville3, Pedro Cózar2, Javier Sanz-López4, Ismael Coronado5, Felipe González6, Ismail Said1 and Mohamed El Houicha7 Abstract A new early Visean coral assemblage has been recorded from turbidite facies in the southern part of the Azrou- Khenifra Basin, northwest of Khenifra, central Morocco. The newly discovered Ba Moussa West (BMW) coral fauna includes Siphonophyllia khenifrense sp. nov., Sychnoelasma urbanowitschi, Cravenia lamellata, Cravenia tela, Cravenia rhytoides, Turnacipora megastoma and Pleurosiphonella crustosa. The early Visean age of the coral assemblage is supported by foraminiferal and conodont data, with the recognition of the basal Visean MFZ9 Zone. This confirms that the first transgression in the Azrou-Khenifra Basin was during the earliest Visean. The allochthonous coral assemblage was recovered from coarse-grained proximal limestone debris flow and turbidite beds within a fault- bounded unit, lying to the west of a thrust syncline containing upper Visean limestones. No evidence exists of the former early Visean shallow-water platform from which the corals were derived. All other in situ platform carbonate rocks around the southern margin of the Azrou-Khenifra Basin are probably of late Visean (Asbian–Brigantian) age. The early Visean Ba Moussa West coral fauna can be compared with that at Tafilalt in eastern Morocco, as well as in other Saharian basins of Algeria. Many of the genera and species in the Ba Moussa West assemblage are identical to those in NW Europe, with which it must have had marine connections. -

A New Trachypsammiid Cnidarian from the Late Permian of Spitsbergen
POLISH POLAR RESEARCH 18 3-4 159-169 1997 Aleksander NOWIŃSKI Institute of Paleobiology Polish Academy of Sciences Twarda 51/55 00-818 Warszawa, POLAND A new trachypsammiid cnidarian from the Late Permian of Spitsbergen ABSTRACT: Starostinella nordica gen. et sp. n. is described from the uppermost Permian (Kapp Starostin Formation) of the Kapp Starostin (Isfjorden) in West Spitsbergen. The new genus is attributed to Trachypsammiidae Gerth - a family incertae sedis among Cnidaria. Members of the Trachypsammiidae have been previously associated with different higher rank taxa within the Cnidaria, or their skeletons were interpreted as a result of symbiosis of a cladochonoidal organism (Tabulata) with an indeterminate hydroid or stromatoporoid. S. nordica gen. et sp. n. seems to support the latter assumption. Hydrocoralla of S. nordica have a simpler structure than those of other Trachypsammiidae and are branching like those of Cladochonus. Their thick-walled, horn-shaped hydrocorallites are surrounded with a very thick cortical zone of sclerenchyme organized into trabecular microstructure. The proper corallite wall is fibro-radial in structure, sharply distinct from the outer cortical zone. Key words: Arctic, Permian, Cnidaria (Trachypsammiidae). Introduction The family Trachypsammiidae Gerth, to which the new genus Starostinella is here assigned, occupies an incertae sedis position among the Cnidaria. Its representatives have been variously included into Tabulata (Favositida, Aulo- porida), Octocorallia (Trachypsammiacea), or simply to Cnidaria without further indication of class nor order. A hypothesis was also put forward that the hydro coralla of Trachypsammiidae result from symbiosis of two skeletal organisms: a cladochonoidal organism (Tabulata) and an indeterminate stromatoporoid or a hydroid. -
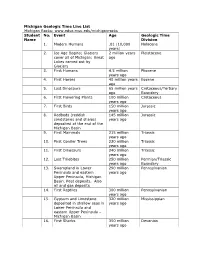
Michigan Geologic Time Line List Michigan Rocks: Student No
Michigan Geologic Time Line List Michigan Rocks: www.educ.msu.edu/michiganrocks Student No. Event Age Geologic Time Name Division 1. Modern Humans .01 (10,000 Holocene years) 2. Ice Age Begins; Glaciers 2 million years Pleistocene cover all of Michigan; Great ago Lakes carved out by Glaciers 3. First Humans 4.5 million Pliocene years ago 4. First Horses 40 million years Eocene ago 5. Last Dinosaurs 65 million years Cretaceous/Tertiary ago Boundary 6. First Flowering Plants 100 million Cretaceous years ago 7. First Birds 150 million Jurassic years ago 8. Redbeds (reddish 145 million Jurassic sandstones and shales) years ago deposited at the end of the Michigan Basin 9. First Mammals 215 million Triassic years ago 10. First Conifer Trees 230 million Triassic years ago 11. First Dinosaurs 240 million Triassic years ago 12. Last Trilobites 250 million Permian/Triassic years ago Boundary 13. Swampland in Lower 290 million Pennsylvanian Peninsula and eastern years ago Upper Peninsula, Michigan Basin. Peat deposits. Also oil and gas deposits 14. First Reptiles 300 million Pennsylvanian years ago 15. Gypsum and Limestone 320 million Mississippian deposited in shallow seas in years ago Lower Peninsula and eastern Upper Peninsula – Michigan Basin 16. First Sharks 350 million Devonian years ago Student No. Event Age Geologic Name Time Division 17. Hexagonaria coral deposits 360 million years Devonian (becomes MI state fossil aka ago Petosky Stone) in Michigan Basin 18. First Amphibians 370 million years Devonian ago 19. First Insects 400 million years Devonian ago 20. First Land Plants 420 million years Ordovician ago 21. -

University of Michigan University Library
CONTRIBUTIONS FROM THE MUSEUM OF PALEONTOLOGY THE UNIVERSITY OF MICHIGAN VOL.23, NO.5, p. 81-91, (4 pls.) JUNE 19, 1970 CORALS OF THE TRAVERSE GROUP OF MICHIGAN PART 13, HEXAGONARIA ERWIN C. STUMM MUSEUM OF PALEONTOLOGY THE UNIVERSITY OF MICHIGAN ANN ARBOR CONTRIBUTIONS FROM THE MUSEUM OF PALEONTOLOGY Director: ROBERTV. KESLING The series of contributions from the Museum of Paleontology is a medium for the publication of papers based chiefly upon the collection in the Museum. When the number of pages issued is sufficient to make a volume, a title page and a table of contents will be sent to libraries on the mailing list, and to individuals upon request. A list of the separate papers may also be obtained. Correspondence should be directed to the Museum of Paleontology, The University of Michigan, Ann Arbor, Michigan 48104. VOLS.2-22. Parts of volumes may be obtained if available. Price lists available upon inquiry. 1. The rodents from the Hagerman local fauna, Upper Pliocene of Idaho, by Richard J. Zakrzewski. Pages 1-36, with 13 text-figures. 2. A new brittle-star from the Middle Devonian Arkona Shale of Ontario, by Robert V. Kesling. Pages 37-51, with 6 plates and 2 text-figures. 3. Phyllocarid crustaceans from the Middle Devonian Silica Shale of northwestern Ohio and southeastern Michigan, by Erwin C. Stumm and Ruth B. Chilman. Pages 53-71, with 7 plates and 4 text-figures. 4. Drepanaster wrighti, a new species of brittle-star from the Middle Devonian Arkona Shale of Ontario, by Robert V. Kesling. Pages 73-79, with 2 plates. -

CNIDARIA Corals, Medusae, Hydroids, Myxozoans
FOUR Phylum CNIDARIA corals, medusae, hydroids, myxozoans STEPHEN D. CAIRNS, LISA-ANN GERSHWIN, FRED J. BROOK, PHILIP PUGH, ELLIOT W. Dawson, OscaR OcaÑA V., WILLEM VERvooRT, GARY WILLIAMS, JEANETTE E. Watson, DENNIS M. OPREsko, PETER SCHUCHERT, P. MICHAEL HINE, DENNIS P. GORDON, HAMISH J. CAMPBELL, ANTHONY J. WRIGHT, JUAN A. SÁNCHEZ, DAPHNE G. FAUTIN his ancient phylum of mostly marine organisms is best known for its contribution to geomorphological features, forming thousands of square Tkilometres of coral reefs in warm tropical waters. Their fossil remains contribute to some limestones. Cnidarians are also significant components of the plankton, where large medusae – popularly called jellyfish – and colonial forms like Portuguese man-of-war and stringy siphonophores prey on other organisms including small fish. Some of these species are justly feared by humans for their stings, which in some cases can be fatal. Certainly, most New Zealanders will have encountered cnidarians when rambling along beaches and fossicking in rock pools where sea anemones and diminutive bushy hydroids abound. In New Zealand’s fiords and in deeper water on seamounts, black corals and branching gorgonians can form veritable trees five metres high or more. In contrast, inland inhabitants of continental landmasses who have never, or rarely, seen an ocean or visited a seashore can hardly be impressed with the Cnidaria as a phylum – freshwater cnidarians are relatively few, restricted to tiny hydras, the branching hydroid Cordylophora, and rare medusae. Worldwide, there are about 10,000 described species, with perhaps half as many again undescribed. All cnidarians have nettle cells known as nematocysts (or cnidae – from the Greek, knide, a nettle), extraordinarily complex structures that are effectively invaginated coiled tubes within a cell. -

Geology of Michigan and the Great Lakes
35133_Geo_Michigan_Cover.qxd 11/13/07 10:26 AM Page 1 “The Geology of Michigan and the Great Lakes” is written to augment any introductory earth science, environmental geology, geologic, or geographic course offering, and is designed to introduce students in Michigan and the Great Lakes to important regional geologic concepts and events. Although Michigan’s geologic past spans the Precambrian through the Holocene, much of the rock record, Pennsylvanian through Pliocene, is miss- ing. Glacial events during the Pleistocene removed these rocks. However, these same glacial events left behind a rich legacy of surficial deposits, various landscape features, lakes, and rivers. Michigan is one of the most scenic states in the nation, providing numerous recre- ational opportunities to inhabitants and visitors alike. Geology of the region has also played an important, and often controlling, role in the pattern of settlement and ongoing economic development of the state. Vital resources such as iron ore, copper, gypsum, salt, oil, and gas have greatly contributed to Michigan’s growth and industrial might. Ample supplies of high-quality water support a vibrant population and strong industrial base throughout the Great Lakes region. These water supplies are now becoming increasingly important in light of modern economic growth and population demands. This text introduces the student to the geology of Michigan and the Great Lakes region. It begins with the Precambrian basement terrains as they relate to plate tectonic events. It describes Paleozoic clastic and carbonate rocks, restricted basin salts, and Niagaran pinnacle reefs. Quaternary glacial events and the development of today’s modern landscapes are also discussed. -

Michigan Fossils Side by Side
MMiicchhiiggaann FFoossssiillss A2 - BRACHIOPOD, B2 - CRINOID pieces, A1 - BRACHIOPOD, B1 - CORAL or CHAIN invertebrate - invertebrate - invertebrate - CORAL, invertebrate - Mucrospifier profundus - Megistocrinus sp. ? - Pentamerus sp. - Cordell Halysites sp. - Cordell Silica formation - Alpena limestone - dolomite - Silurian age - dolomite - Silurian age - Devonian age - Devonian age - Alpena Chippewa Co. - 65 mm, Chippewa Co. - 110 mm, Washtenaw Co. - 50 mm, Co. - 30 mm (lower right) internal cast or steinkern siliceous replacement - calcite replacement - R. , calcite replacement - - R. Elowski R. Milstein Milstein GSD B4 - CORAL or A4 - CORAL or B3 - SNAIL or COLONIAL CORAL A3 - TRILOBITE, COLONIAL CORAL, GASTROPOD, Petoskey Stone, invertebrate - Phacops invertebrate - invertebrate - genus not invertebrate - rana - Silica formation - Syringopora sp. - Cordell determined - Cordell Hexagonaria percarinata Devonian age - dolomite - Silurian age - dolomite - Silurian age - - Alpena Limestone - Washtenaw Co. - 70 mm, s Chippewa Co. - 125 mm, s Chippewa Co. - 75 mm, Devonian age - calcite replacement - S. siliceous replacement - l internal cast or steinkern Charlevoix Co. - 200 mm, Wilson l R. Milstein - GSD calcite replacement - R. i i Milstein s s D1 - BANDED IRON C1 - MASTODON Tooth, C2 - EUCARYOTIC algae FORMATION (BIF), from D2 - PLANT root section, s vertebrate - Mammut filaments, plant - s fossils - result of plant - Stigmaria, genus americanum - Glacial Grypania spiralis - Grypania and others - not determined - Saginaw deposit - Quaternary age Negaunee Iron Formation Banded Iron Formation - Formation - o - 200 mm long, the - Precambrian age - o Precambrian age - Pennsylvanian age - “Michigan State Fossil” - Marquette Co. - large Marquette Co. - 600 mm, Eaton Co. - 150 mm in Central Michigan loop about 20 mm , F from the rock walkway at diameter, internal cast - F University Rowe Museum oldest macrofossil - GSD the - Eddy Discovery T. -

Newsletter Number 80
The Palaeontology Newsletter Contents 80 Editorial 2 Association Business 3 News 16 Association Meetings 19 From our correspondents The very Dickens of a palaeontologist 23 PalaeoMath 101: Round the Bend … 32 Future meetings of other bodies 49 Meeting Report British Ecological Society Macro-SIG 57 Reporter: A fossil-fuelled future? 60 Encouraging palaeontology in schools 63 James Mckay – palaeo artist 75 Caithness fish on Edinburgh street 79 Palaeontology vol 55 parts 3 & 4 82–83 SPP 87: Tabulate Corals in Poland 84 Reminder: The deadline for copy for Issue no 81 is 3rd November 2012. On the Web: <http://www.palass.org/> ISSN: 0954-9900 Newsletter 80 2 Editorial Summer is upon us, whatever that means for you. For me in Scotland it is the long hours of daylight and the chance to get round lots of mountaintops in a day and collect fossils in better light than usual. As the short report about Ken Shaw’s fossil fish find in a paving slab in the heart of Edinburgh shows, sometimes exciting finds await us in rather unexpected places. For others, school is out – but Gordon Neighbour’s article on palaeontology and schools reminds us that we should be looking to what we can do to help encourage school pupils to engage with palaeontology. Although Liam Herringshaw’s somewhat downbeat article about the lack of retention of post-Ph.D. palaeontologists by UK universities and other institutions may have those pupils asking why they should focus on palaeontology. The analytical palaeobiologist in me would ask immediately whether other “clades” of Earth Scientists are having a similarly hard time of it. -

Progress to Extinction: Increased Specialisation Causes the Demise of Animal Clades Recei�E�: �3 �O�Em�Er �015 P
www.nature.com/scientificreports OPEN Progress to extinction: increased specialisation causes the demise of animal clades receie: 3 oemer 015 P. Raia1, F. Carotenuto1, A. Mondanaro1, S. Castiglione1, F. Passaro1, F. Saggese1, accepte: 1 u 016 M. Melchionna1, C. Serio1, L. Alessio1, D. Silvestro2 & M. Fortelius3,4 Puise: 10 uust 016 Animal clades tend to follow a predictable path of waxing and waning during their existence, regardless of their total species richness or geographic coverage. Clades begin small and undiferentiated, then expand to a peak in diversity and range, only to shift into a rarely broken decline towards extinction. While this trajectory is now well documented and broadly recognised, the reasons underlying it remain obscure. In particular, it is unknown why clade extinction is universal and occurs with such surprising regularity. Current explanations for paleontological extinctions call on the growing costs of biological interactions, geological accidents, evolutionary traps, and mass extinctions. While these are efective causes of extinction, they mainly apply to species, not clades. Although mass extinctions is the undeniable cause for the demise of a sizeable number of major taxa, we show here that clades escaping them go extinct because of the widespread tendency of evolution to produce increasingly specialised, sympatric, and geographically restricted species over time. Most animal clades follow a predictable path in geographic commonness and taxonomic diversity over time1,2. Clades usually start within a very restricted range3, and then expand and diversify to occupy large stretches of Earth. Almost immediately afer this peak in success, they start declining in diversity and fnally go extinct1,2,4–6 (although a few may survive with as little diversity as one genus like sphenodonts, coelacanths, and nautiloids). -

Carboniferous Tabulate Corals from the Sardar Formation in the Ozbak-Kuh Mountains, East-Central Iran
Bull. Natl. Mus. Nat. Sci., Ser. C, 46, pp. 47–59, December 25, 2020 Carboniferous Tabulate Corals from the Sardar Formation in the Ozbak-kuh Mountains, East-Central Iran Shuji Niko1* and Mahdi Badpa2 1 Department of Environmental Studies, Faculty of Integrated Arts and Sciences, Hiroshima University, Higashihiroshima, Hiroshima 739–8521, Japan 2 Department of Geology, Payame Noor University of Qom, Qom, Iran *Author for correspondence: [email protected] Abstract Five species of tabulate corals are described from the Serpukhovian–Bashkirian (late early– early late Carboniferous) part of the Sardar Formation in the Ozbak-kuh Mountains, East-Central Iran. They are Michelinia flugeli sp. nov., late Bashkirian, Michelinia sp. indet., late Bashkirian, Donetzites mariae Flügel, 1975, late Serpukhovian, Syringopora iranica sp. nov., late Serpukhovian, and Multithe- copora sp. cf. M. huanglungensis Lee and Chu in Lee et al., 1930, late Serpukhovian to late Bashkirian. The Sardar tabulate coral fauna occurs from limestones that formed in Peri-Gondwana at the low lati- tudes of the southern hemisphere and faced the Paleo-Tethys Ocean. Except for a cosmopolitan species, closely related to comparable species with the fauna are reported from the Akiyoshi seamount chain, the South China Continent, and the Australian eastern Gondwana. Key words: Serpukhovian–Bashkirian, late early–early late Carboniferous, Favositida, Auloporida, Peri-Gondwana Introduction et al. (2006) separated its upper part from the for- mation as the Zaladu Formation that is correlative The Ozbak-kuh Mountains are a fault topography with the Gzhelian (late late Carboniferous) to Asse- in the Kashmar-Kerman Tectonic Zone (Stöcklin lian (early early Permian).