Histology of a Freshwater Mussel
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

Common Name: Chiton Class: Polyplacophora
Common Name: Chiton Class: Polyplacophora Scrapes algae off rock with radula 8 Overlapping Plates Phylum? Mollusca Class? Gastropoda Common name? Brown sea hare Class? Scaphopoda Common name? Tooth shell or tusk shell Mud Tentacle Foot Class? Gastropoda Common name? Limpet Phylum? Mollusca Class? Bivalvia Class? Gastropoda Common name? Brown sea hare Phylum? Mollusca Class? Gastropoda Common name? Nudibranch Class? Cephalopoda Cuttlefish Octopus Squid Nautilus Phylum? Mollusca Class? Gastropoda Most Bivalves are Filter Feeders A B E D C • A: Mantle • B: Gill • C: Mantle • D: Foot • E: Posterior adductor muscle I.D. Green: Foot I.D. Red Gills Three Body Regions 1. Head – Foot 2. Visceral Mass 3. Mantle A B C D • A: Radula • B: Mantle • C: Mouth • D: Foot What are these? Snail Radulas Dorsal HingeA Growth line UmboB (Anterior) Ventral ByssalC threads Mussel – View of Outer Shell • A: Hinge • B: Umbo • C: Byssal threads Internal Anatomy of the Bay Mussel A B C D • A: Labial palps • B: Mantle • C: Foot • D: Byssal threads NacreousB layer Posterior adductorC PeriostracumA muscle SiphonD Mantle Byssal threads E Internal Anatomy of the Bay Mussel • A: Periostracum • B: Nacreous layer • C: Posterior adductor muscle • D: Siphon • E: Mantle Byssal gland Mantle Gill Foot Labial palp Mantle Byssal threads Gill Byssal gland Mantle Foot Incurrent siphon Byssal Labial palp threads C D B A E • A: Foot • B: Gills • C: Posterior adductor muscle • D: Excurrent siphon • E: Incurrent siphon Heart G F H E D A B C • A: Foot • B: Gills • C: Mantle • D: Excurrent siphon • E: Incurrent siphon • F: Posterior adductor muscle • G: Labial palps • H: Anterior adductor muscle Siphon or 1. -

Silicified Eocene Molluscs from the Lower Murchison District, Southern Carnarvon Basin, Western Australia
[<ecords o{ the Western A uslralian Museum 24: 217--246 (2008). Silicified Eocene molluscs from the Lower Murchison district, Southern Carnarvon Basin, Western Australia Thomas A. Darragh1 and George W. Kendrick2.3 I Department of Invertebrate Palaeontology, Museum Victoria, 1'.0. Box 666, Melbourne, Victoria 3001, Australia. Email: tdarragh(il.Illuseum.vic.gov.au :' Department of Earth and Planetary Sciences, Western Australian Museum, Locked Bag 49, Welshpool D.C., Western Australia 6986, Australia. 1 School of Earth and Ceographical Sciences, The University of Western Australia, 35 Stirling Highway, Crawlev, Western Australia 6009, Australia. Abstract - Silicified Middle to Late Eocene shallow water sandstones outcropping in the Lower Murchison District near Kalbarri township contain a silicified fossil fauna including foraminifera, sponges, bryozoans, solitary corals, brachiopods, echinoids and molluscs. The known molluscan fauna consists of 51 species, comprising 2 cephalopods, 14 bivalves, 1 scaphopod and 34 gastropods. Of these taxa three are newly described, Cerithium lvilya, Zeacolpus bartol1i, and Lyria lamellatoplicata. 25 of these molluscs are identical to or closely comparable with taxa from the southern Australian Eocene. The occurrence of this fauna extends the Southeast Australian Province during the Eocene from southwest Western Australia along the west coast north to at least 27° present day south latitude; consequently the province is here renamed the Southern Australian Province. Keywords: siliceous fossils, Eocene, Kalbarri, molluscs, new taxa, Carnarvon Basin, biogeography, Southern Australian Province. INTRODUCTION The source deposit, a pallid to ferruginous silicified Eocene marine molluscan assemblages from sandstone, forms a weakly defined, low breakaway coastal sedimentary basins in southern Australia trending N-S and sloping gently westward. -
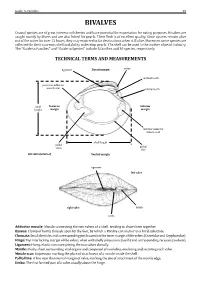
Field Identification Guide to the Living Marine Resources In
Guide to Families 29 BIVALVES Coastal species are of great interest to fisheries and have potential for exportation for eating purposes. Bivalves are caught mainly by divers and are also fished for pearls. Their flesh is of excellent quality. Since oysters remain alive out of the water for over 12 hours, they may exported to far destinations when still alive. Moreover, some species are collected for their nacreous shell and ability to develop pearls. The shell can be used in the mother of pearl industry. The “Guide to Families’’ andTECHNICAL ‘‘Guide to Species’’ TERMS include 5AND families MEASUREMENTS and 10 species, respectively. ligament Dorsal margin umbo posterior adductor cardinal tooth muscle scar lateral tooth Posterior Anterior margin margin shell height anterior adductor muscle scar pallial sinus pallial shell length line left valve (interior) Ventral margin ligament left valve right valve lunule umbo Adductor muscle: Byssus: Chomata: Muscle connecting the two valves of a shell, tending to draw them together. Hinge: Clump of horny threads spun by the foot, by which a Bivalve can anchor to a hard substrate. Ligament: Small denticles and corresponding pits located on the inner margin of the valves (Ostreidae and Gryphaeidae). Mantle: Top interlocking margin of the valves, often with shelly projections (teeth) and corresponding recesses (sockets). Muscle scar: Horny, elastic structure joining the two valves dorsally. Pallial line: Fleshy sheet surrounding vital organs and composed of two lobes, one lining and secreting each valve. Umbo: Impression marking the place of attachment of a muscle inside the shell. A line near the internal margin of valve, marking the site of attachment of the mantle edge. -

Clam Dissection Guideline
Clam Dissection Guideline BACKGROUND: Clams are bivalves, meaning that they have shells consisting of two halves, or valves. The valves are joined at the top, and the adductor muscles on each side hold the shell closed. If the adductor muscles are relaxed, the shell is pulled open by ligaments located on each side of the umbo. The clam's foot is used to dig down into the sand, and a pair of long incurrent and excurrent siphons that extrude from the clam's mantle out the side of the shell reach up to the water above (only the exit points for the siphons are shown). Clams are filter feeders. Water and food particles are drawn in through one siphon to the gills where tiny, hair-like cilia move the water, and the food is caught in mucus on the gills. From there, the food-mucus mixture is transported along a groove to the palps (mouth flaps) which push it into the clam's mouth. The second siphon carries away the water. The gills also draw oxygen from the water flow. The mantle, a thin membrane surrounding the body of the clam, secretes the shell. The oldest part of the clam shell is the umbo, and it is from the hinge area that the clam extends as it grows. I. Purpose: The purpose of this lab is to identify the internal and external structures of a mollusk by dissecting a clam. II. Materials: 2 pairs of safety goggles 1 paper towel 2 pairs of gloves 1 pair of scissors 1 preserved clam 2 pairs of forceps 1 dissecting tray 2 probes III. -

On the Origin, Nature, and Function of the Crystalline Style of Lamellibranchsi Thurlow C
AUTHOR’S AFJSTRA~OF THIB PAPER IESUED BY THE BIBLIOQRAPEIC BERVICE, APRIL 20 ON THE ORIGIN, NATURE, AND FUNCTION OF THE CRYSTALLINE STYLE OF LAMELLIBRANCHSI THURLOW C. NELSON From the Zoological Laboratory 01 the University of Wisconsin SEVENTEEN FIGURES CONTENTS I. Introduction ........................................................... 53 The crystalline style.. ................................................ 54 Historical.. ... ............................................ 57 Materials and methods.. ............................................. 63 ................................... 65 g organs ........................... 65 ................................... 71 ................................... 73 Histology of the style sac.. ............................................ 74 The ciliary mechanism ............................... 76 The secretion and fo le .............................. 80 Embryology of the style-bearing organs.. ............................. 87 3. Nature .............................................. 89 Description of the style.. .................... .................... 89 Composition of the style.. ........ ............... 90 Nature of the gastric shield.. ............... 96 The Spirochaetes of the cryst ............... 97 4. Function ....................... ............... 98 5. Summary and conclusions...... ............................... 107 Bibliography. ........... ........................................... 108 1. INTRODUCTION “What has not been written concerning the crystallinestyle, and in how many ways -

Production of the Snail Oxytrema Silicula (Gould) in an Experimental Stream
AN ABSTRACT OF TIlE THESIS OF Russell David Earnest for the M. S. (Name of student) (Degree) in Fisheries presented on February 22,1967 (Major) (Date) Title. Production of the Snail Oxytrema silicula (Gould) in an ExDerimental Stream Abstract approved Redacted for Privacy Gerald F. Davis A study was performed of the influence of enrichment on the production and food relations of the stream snail, Oxytrema silicula, in the Berry Creek experimental stream from October, 1963 through February, 1965.Four experimental stream sections that were separated from each other by screens were used in thetudy.Two upstream sections (I and II) were not enriched; receiving energy only from sunlight and natural allochthonous materials.Two down- stream sections (III and TV) w're continuously enriched with sucrose and urea. As aresult,growthsof the bacterium Sphaerotilus natans developed on therifflesof thesesections.The removal of trees from alongside Sections II and IV resulted in smaller quantities of leaves and more sunlight reaching these sections than reached Sec- tions I and III. Snails were removed monthly froma0. 26m2area of riffle of each section and were separated into different size-groups.The groups were considered to represent the 1959, 1960, 1961, 1962, 1963, and 1964 year-classes.Production for each age-class was calculated from measurements of growth rate and average biomass. Aquarium experiments with snails of sizes similar to those of the 1961 year-class, which were the most abundant in the stream, established relationships between rates of growth and foodconsump- tion during two seasonal periods.Estimates of annual total food consumption in each section were based upon these relationships and upon knowledge of the growth rates of 1961 year-class snails and of the total snail biomass of all age-classes. -

Copyrighted Material
319 Index a oral cavity 195 guanocytes 228, 231, 233 accessory sex glands 125, 316 parasites 210–11 heart 235 acidophils 209, 254 pharynx 195, 197 hemocytes 236 acinar glands 304 podocytes 203–4 hemolymph 234–5, 236 acontia 68 pseudohearts 206, 208 immune system 236 air sacs 305 reproductive system 186, 214–17 life expectancy 222 alimentary canal see digestive setae 191–2 Malpighian tubules 232, 233 system taxonomy 185 musculoskeletal system amoebocytes testis 214 226–9 Cnidaria 70, 77 typhlosole 203 nephrocytes 233 Porifera 28 antennae nervous system 237–8 ampullae 10 Decapoda 278 ocelli 240 Annelida 185–218 Insecta 301, 315 oral cavity 230 blood vessels 206–8 Myriapoda 264, 275 ovary 238 body wall 189–94 aphodus 38 pedipalps 222–3 calciferous glands 197–200 apodemes 285 pharynx 230 ciliated funnel 204–5 apophallation 87–8 reproductive system 238–40 circulatory system 205–8 apopylar cell 26 respiratory system 236–7 clitellum 192–4 apopyle 38 silk glands 226, 242–3 coelomocytes 208–10 aquiferous system 21–2, 33–8 stercoral sac 231 crop 200–1 Arachnida 221–43 sucking stomach 230 cuticle 189 biomedical applications 222 taxonomy 221 diet 186–7 body wall 226–9 testis 239–40 digestive system 194–203 book lungs 236–7 tracheal tube system 237 dissection 187–9 brain 237 traded species 222 epidermis 189–91 chelicera 222, 229 venom gland 241–2 esophagus 197–200 circulatory system 234–6 walking legs 223 excretory system 203–5 COPYRIGHTEDconnective tissue 228–9 MATERIALzoonosis 222 ganglia 211–13 coxal glands 232, 233–4 archaeocytes 28–9 giant nerve -

Freshwater Mussels of Maritime Canada: a Flashcard Guide
Freshwater Mussels of Maritime Canada: A Flashcard Guide In Wolastoqey, Mi’kmaw, French and English UMBO DORSAL MARGIN ANTERIOR POSTERIOR MARGIN MARGIN VENTRAL MARGIN Donald F. McAlpine, Mary C. Sollows, Jacqueline B. Madill and André L. Martel ISBN 978-0-919326-80-4 All photos copyright the Canadian Museum of Nature Acknowledgements: Funding for this publication provided by the Department of Fisheries and Oceans and the New Brunswick Museum. Special thanks to Ree Brennin Houston, Department of Fisheries and Oceans; Anne Hamilton, Brent Suttie, New Brunswick Archaeological Services Branch, and indigenous language translators Allan Tremblay (Wolastoqiyk), George Paul, Howard Augustine, and Karen Narvey (Mi’kmaw). Citation: McAlpine, D.F., M.C. Sollows, J. B. Madill, and A. L. Martel. 2018. Freshwater Mussels of Maritime Canada: A Flashcard Guide in Wolastoqey, Mi’kmaw, French and English. New Brunswick Museum, Saint John, New Brunswick, and Canadian Museum of Nature, Ottawa, Canada. Use in conjunction with Martel, A. L.,D.F. McAlpine, J. Madill, D. Sabine, A. Paquet, M. Pulsifer and M. Elderkin. 2010. Pp. 551-598. Freshwater Mussels (Bivalvia: Margaritiferidae, Unionidae) of the Atlantic Maritime Ecozone. In D.F. McAlpine and I.M. Smith (eds.). Assessment of Species Diversity in the Atlantic Maritime Ecozone. NRC Research Press, National Research Council of Canada, Ottawa, ON. 785 pp. Nedeau, E.J., M.A. McCollough, and B.I. Swartz. 2000. Freshwater Mussels of Maine. Maine Department of Inland Fisheries and Wildlife, Augusta, ME, 118 pp. -

Pleistocene Molluscs from the Namaqualand Coast
ANNALS OF THE SOUTH AFRICAN MUSEUM ANNALE VAN DIE SUID-AFRIKAANSE MUSEUM Volume 52 Band July 1969 Julie Part 9 Dee! PLEISTOCENE MOLLUSCS FROM THE NAMAQUALAND COAST By A.J.CARRINGTON & B.F.KENSLEY are issued in parts at irregular intervals as material becomes available Obtainable from the South African Museum, P.O. Box 61, Cape Town word uitgegee in dele opongereelde tye na beskikbaarheid van stof OUT OF PRINT/UIT nRUK I, 2(1, 3, 5, 7-8), 3(1-2, 5, t.-p.i.), 5(2, 5, 7-9), 6(1, t.-p.i.), 7(1, 3), 8, 9(1-2), 10(1-3), 11(1-2, 7, t.-p.i.), 21, 24(2), 27, 31(1-3), 38, 44(4)· Price of this part/Prys van hierdie deel Rg.oo Trustees of the South African Museum © 1969 Printed in South Africa by In Suid-Afrika gedruk deur The Rustica Press, Pty., Ltd. Die Rustica-pers, Edms., Bpk. Court Road, Wynberg, Cape Courtweg, Wynberg, Kaap By A. ]. CARRINGTON & B. F. KENSLEY South African Museum, Cape Town (With plates 18 to 29 and I I figures) PAGE Introduction 189 Succession 190 Systematic discussion. 191 Acknowledgements 222 Summary. 222 References 223 INTRODUCTION In the course of an examination of the Tertiary to Recent sediments of the Namaqualand coast, being carried out by one of the authors (A.].C.), a collection of fossil molluscs was assembled from the Pleistocene horizons encountered in the area. The purpose of this paper is to introduce and describe some twenty species from this collection, including forms new to the South Mrican palaeontological literature. -

Mollusca, Archaeogastropoda) from the Northeastern Pacific
Zoologica Scripta, Vol. 25, No. 1, pp. 35-49, 1996 Pergamon Elsevier Science Ltd © 1996 The Norwegian Academy of Science and Letters Printed in Great Britain. All rights reserved 0300-3256(95)00015-1 0300-3256/96 $ 15.00 + 0.00 Anatomy and systematics of bathyphytophilid limpets (Mollusca, Archaeogastropoda) from the northeastern Pacific GERHARD HASZPRUNAR and JAMES H. McLEAN Accepted 28 September 1995 Haszprunar, G. & McLean, J. H. 1995. Anatomy and systematics of bathyphytophilid limpets (Mollusca, Archaeogastropoda) from the northeastern Pacific.—Zool. Scr. 25: 35^9. Bathyphytophilus diegensis sp. n. is described on basis of shell and radula characters. The radula of another species of Bathyphytophilus is illustrated, but the species is not described since the shell is unknown. Both species feed on detached blades of the surfgrass Phyllospadix carried by turbidity currents into continental slope depths in the San Diego Trough. The anatomy of B. diegensis was investigated by means of semithin serial sectioning and graphic reconstruction. The shell is limpet like; the protoconch resembles that of pseudococculinids and other lepetelloids. The radula is a distinctive, highly modified rhipidoglossate type with close similarities to the lepetellid radula. The anatomy falls well into the lepetelloid bauplan and is in general similar to that of Pseudococculini- dae and Pyropeltidae. Apomorphic features are the presence of gill-leaflets at both sides of the pallial roof (shared with certain pseudococculinids), the lack of jaws, and in particular many enigmatic pouches (bacterial chambers?) which open into the posterior oesophagus. Autapomor- phic characters of shell, radula and anatomy confirm the placement of Bathyphytophilus (with Aenigmabonus) in a distinct family, Bathyphytophilidae Moskalev, 1978. -

Structure and Function of the Digestive System in Molluscs
Cell and Tissue Research (2019) 377:475–503 https://doi.org/10.1007/s00441-019-03085-9 REVIEW Structure and function of the digestive system in molluscs Alexandre Lobo-da-Cunha1,2 Received: 21 February 2019 /Accepted: 26 July 2019 /Published online: 2 September 2019 # Springer-Verlag GmbH Germany, part of Springer Nature 2019 Abstract The phylum Mollusca is one of the largest and more diversified among metazoan phyla, comprising many thousand species living in ocean, freshwater and terrestrial ecosystems. Mollusc-feeding biology is highly diverse, including omnivorous grazers, herbivores, carnivorous scavengers and predators, and even some parasitic species. Consequently, their digestive system presents many adaptive variations. The digestive tract starting in the mouth consists of the buccal cavity, oesophagus, stomach and intestine ending in the anus. Several types of glands are associated, namely, oral and salivary glands, oesophageal glands, digestive gland and, in some cases, anal glands. The digestive gland is the largest and more important for digestion and nutrient absorption. The digestive system of each of the eight extant molluscan classes is reviewed, highlighting the most recent data available on histological, ultrastructural and functional aspects of tissues and cells involved in nutrient absorption, intracellular and extracellular digestion, with emphasis on glandular tissues. Keywords Digestive tract . Digestive gland . Salivary glands . Mollusca . Ultrastructure Introduction and visceral mass. The visceral mass is dorsally covered by the mantle tissues that frequently extend outwards to create a The phylum Mollusca is considered the second largest among flap around the body forming a space in between known as metazoans, surpassed only by the arthropods in a number of pallial or mantle cavity. -

The Freshwater Bivalve Mollusca (Unionidae, Sphaeriidae, Corbiculidae) of the Savannah River Plant, South Carolina
SRQ-NERp·3 The Freshwater Bivalve Mollusca (Unionidae, Sphaeriidae, Corbiculidae) of the Savannah River Plant, South Carolina by Joseph C. Britton and Samuel L. H. Fuller A Publication of the Savannah River Plant National Environmental Research Park Program United States Department of Energy ...---------NOTICE ---------, This report was prepared as an account of work sponsored by the United States Government. Neither the United States nor the United States Depart mentof Energy.nor any of theircontractors, subcontractors,or theiremploy ees, makes any warranty. express or implied or assumes any legalliabilityor responsibilityfor the accuracy, completenessor usefulnessofanyinformation, apparatus, product or process disclosed, or represents that its use would not infringe privately owned rights. A PUBLICATION OF DOE'S SAVANNAH RIVER PLANT NATIONAL ENVIRONMENT RESEARCH PARK Copies may be obtained from NOVEMBER 1980 Savannah River Ecology Laboratory SRO-NERP-3 THE FRESHWATER BIVALVE MOLLUSCA (UNIONIDAE, SPHAERIIDAE, CORBICULIDAEj OF THE SAVANNAH RIVER PLANT, SOUTH CAROLINA by JOSEPH C. BRITTON Department of Biology Texas Christian University Fort Worth, Texas 76129 and SAMUEL L. H. FULLER Academy of Natural Sciences at Philadelphia Philadelphia, Pennsylvania Prepared Under the Auspices of The Savannah River Ecology Laboratory and Edited by Michael H. Smith and I. Lehr Brisbin, Jr. 1979 TABLE OF CONTENTS Page INTRODUCTION 1 STUDY AREA " 1 LIST OF BIVALVE MOLLUSKS AT THE SAVANNAH RIVER PLANT............................................ 1 ECOLOGICAL