Official Journal L355
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-
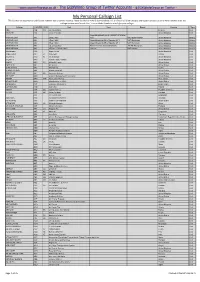
My Personal Callsign List This List Was Not Designed for Publication However Due to Several Requests I Have Decided to Make It Downloadable
- www.egxwinfogroup.co.uk - The EGXWinfo Group of Twitter Accounts - @EGXWinfoGroup on Twitter - My Personal Callsign List This list was not designed for publication however due to several requests I have decided to make it downloadable. It is a mixture of listed callsigns and logged callsigns so some have numbers after the callsign as they were heard. Use CTL+F in Adobe Reader to search for your callsign Callsign ICAO/PRI IATA Unit Type Based Country Type ABG AAB W9 Abelag Aviation Belgium Civil ARMYAIR AAC Army Air Corps United Kingdom Civil AgustaWestland Lynx AH.9A/AW159 Wildcat ARMYAIR 200# AAC 2Regt | AAC AH.1 AAC Middle Wallop United Kingdom Military ARMYAIR 300# AAC 3Regt | AAC AgustaWestland AH-64 Apache AH.1 RAF Wattisham United Kingdom Military ARMYAIR 400# AAC 4Regt | AAC AgustaWestland AH-64 Apache AH.1 RAF Wattisham United Kingdom Military ARMYAIR 500# AAC 5Regt AAC/RAF Britten-Norman Islander/Defender JHCFS Aldergrove United Kingdom Military ARMYAIR 600# AAC 657Sqn | JSFAW | AAC Various RAF Odiham United Kingdom Military Ambassador AAD Mann Air Ltd United Kingdom Civil AIGLE AZUR AAF ZI Aigle Azur France Civil ATLANTIC AAG KI Air Atlantique United Kingdom Civil ATLANTIC AAG Atlantic Flight Training United Kingdom Civil ALOHA AAH KH Aloha Air Cargo United States Civil BOREALIS AAI Air Aurora United States Civil ALFA SUDAN AAJ Alfa Airlines Sudan Civil ALASKA ISLAND AAK Alaska Island Air United States Civil AMERICAN AAL AA American Airlines United States Civil AM CORP AAM Aviation Management Corporation United States Civil -

Reglamento (CE) No 1144/2009 De La Comisión, De 26 De Noviembre De
L 312/16 ES Diario Oficial de la Unión Europea 27.11.2009 REGLAMENTO (CE) N o 1144/2009 DE LA COMISIÓN de 26 de noviembre de 2009 que modifica el Reglamento (CE) no 474/2006, por el que se establece la lista comunitaria de las compañías aéreas objeto de una prohibición de explotación en la Comunidad (Texto pertinente a efectos del EEE) LA COMISIÓN DE LAS COMUNIDADES EUROPEAS, 3922/91, de 16 de diciembre de 1991, relativo a la armonización de normas técnicas y procedimientos ad ministrativos aplicables a la aviación civil ( 3 ). Visto el Tratado constitutivo de la Comunidad Europea, (5) Las autoridades responsables de la supervisión normativa o Visto el Reglamento (CE) n 2111/2005 del Parlamento Euro de las compañías aéreas afectadas fueron también con peo y del Consejo, de 14 de diciembre de 2005, relativo al sultadas por la Comisión y, en algunos casos, por algu establecimiento de una lista comunitaria de las compañías aé nos Estados miembros. reas sujetas a una prohibición de explotación en la Comunidad y a la información que deben recibir los pasajeros aéreos sobre la identidad de la compañía operadora, y por el que se deroga el 1 artículo 9 de la Directiva 2004/36/CE ( ), y, en particular, su (6) El Comité de Seguridad Aérea ha oído las presentaciones artículo 4, de la Agencia Europea de Seguridad Aérea (AESA) y de la Comisión sobre los proyectos de asistencia técnica desa rrollados en países afectados por el Reglamento (CE) o Considerando lo siguiente: n 2111/2005. Se le ha informado de las peticiones de proseguir la asistencia técnica y la cooperación a fin de mejorar la capacidad administrativa y técnica de las au toridades de aviación civil para resolver cualquier incum o (1) El Reglamento (CE) n 474/2006 de la Comisión, de plimiento de las normas internacionales aplicables. -

Commission Implementing Regulation (Eu) 2016
L 160/50 EN Official Journal of the European Union 17.6.2016 COMMISSION IMPLEMENTING REGULATION (EU) 2016/963 of 16 June 2016 amending Regulation (EC) No 474/2006 as regards the list of air carriers which are subject to an operating ban within the Union (Text with EEA relevance) THE EUROPEAN COMMISSION, Having regard to the Treaty on the Functioning of the European Union, Having regard to Regulation (EC) No 2111/2005 of the European Parliament and the Council of 14 December 2005 on the establishment of a Community list of air carriers subject to an operating ban within the Community and on informing air passengers of the identity of the operating carrier, and repealing Article 9 of Directive 2004/36/EC (1), and in particular Article 4(2) thereof, Whereas: (1) Commission Regulation (EC) No 474/2006 (2) established the list of air carriers which are subject to an operating ban within the Union, referred to in Chapter II of Regulation (EC) No 2111/2005. (2) In accordance with Article 4(3) of Regulation (EC) No 2111/2005, certain Member States and the European Aviation Safety Agency (‘EASA’) communicated to the Commission information that is relevant in the context of updating that list. Relevant information was also communicated by third countries and international organ isations. On the basis of that information, the list should be updated. (3) The Commission informed all air carriers concerned, either directly or through the authorities responsible for their regulatory oversight, about the essential facts and considerations which would form the basis for a decision to impose an operating ban on them within the Union or to modify the conditions of an operating ban imposed on an air carrier which is included in the list. -

Uoffisiell Oversettelse
NOR/312R1146.irja OJ L 333/12, p. 7-33 COMMISSION IMPLEMENTING REGULATION (EU) No 1146/2012 of 3 December 2012 amending Regulation (EC) No 474/2006 establishing the Community list of air carriers which are subject to an operating ban within the Community (UOFFISIELL OVERSETTELSE) KOMMISJONENS GJENNOMFØRINGSFORORDNING (EU) nr. 1146/2012 av 3. desember 2012 om endring av forordning (EF) nr. 474/2006 om opprettelse av fellesskapslisten over luftfartsselskaper som er underlagt driftsforbud i Fellesskapet EUROPAKOMMISJONEN HAR — under henvisning til traktaten om Den europeiske unions virkemåte, under henvisning til europaparlaments- og rådsforordning (EF) nr. 2111/2005 av 14. desember 2005 om opprettelse av en fellesskapsliste over luftfartsselskaper underlagt driftsforbud i Fellesskapet og om informasjon til lufttransportpassasjerer om identiteten til utførende luftfartsselskaper, og om oppheving av artikkel 9 i direktiv 2004/36/EF(1), særlig artikkel 4(2), og ut fra følgende betraktninger: 3 1) Ved kommisjonsforordning (EF) nr. 474/2006( ) av 22. mars 2006 ble fellesskapslisten over luftfartsselskaper som er underlagt driftsforbud i Unionen, omhandlet i kapittel II i forordning (EF) nr. 2111/2005, opprettet. 2) I samsvar med artikkel 4 nr. 3 i forordning (EF) nr. 2111/2005 har visse medlemsstater samt Det europeiske flysikkerhetsbyrå (heretter kalt «EASA») underrettet Kommisjonen om opplysninger som er relevante for ajourføringen av fellesskapslisten. Relevante opplysninger er også oversendt av tredjestater. På grunnlag av dette bør fellesskapslisten ajourføres. 3) Kommisjonen har underrettet alle berørte luftfartsselskaper direkte eller, dersom dette ikke var praktisk mulig, gjennom tilsynsmyndighetene, om hvilke sentrale fakta og årsaker som vil bli lagt til grunn for en beslutning om å pålegge dem driftsforbud i Unionen eller endre vilkårene i et driftsforbud for et luftfartsselskap som er oppført på fellesskapslisten. -

1.4. Coding and Decoding of Airlines 1.4.1. Coding Of
1.4. CODING AND DECODING OF AIRLINES 1.4.1. CODING OF AIRLINES In addition to the airlines' full names in alphabetical order the list below also contains: - Column 1: the airlines' prefix numbers (Cargo) - Column 2: the airlines' 2 character designators - Column 3: the airlines' 3 letter designators A Explanation of symbols: + IATA Member & IATA Associate Member * controlled duplication # Party to the IATA Standard Interline Traffic Agreement (see section 8.1.1.) © Cargo carrier only Full name of carrier 1 2 3 40-Mile Air, Ltd. Q5 MLA AAA - Air Alps Aviation A6 LPV AB Varmlandsflyg T9 ABX Air, Inc. © 832 GB Ada Air + 121 ZY ADE Adria Airways + # 165 JP ADR Aegean Airlines S.A. + # 390 A3 AEE Aer Arann Express (Comharbairt Gaillimh Teo) 809 RE REA Aeris SH AIS Aer Lingus Limited + # 053 EI EIN Aero Airlines A.S. 350 EE Aero Asia International Ltd. + # 532 E4 Aero Benin S.A. EM Aero California + 078 JR SER Aero-Charter 187 DW UCR Aero Continente 929 N6 ACQ Aero Continente Dominicana 9D Aero Express Del Ecuador - Trans AM © 144 7T Aero Honduras S.A. d/b/a/ Sol Air 4S Aero Lineas Sosa P4 Aero Lloyd Flugreisen GmbH & Co. YP AEF Aero Republica S.A. 845 P5 RPB Aero Zambia + # 509 Z9 Aero-Condor S.A. Q6 Aero Contractors Company of Nigeria Ltd. AJ NIG Aero-Service BF Aerocaribe 723 QA CBE Aerocaribbean S.A. 164 7L CRN Aerocontinente Chile S.A. C7 Aeroejecutivo S.A. de C.V. 456 SX AJO Aeroflot Russian Airlines + # 555 SU AFL Aeroflot-Don 733 D9 DNV Aerofreight Airlines JSC RS Aeroline GmbH 7E AWU Aerolineas Argentinas + # 044 AR ARG Aerolineas Centrales de Colombia (ACES) + 137 VX AES Aerolineas de Baleares AeBal 059 DF ABH Aerolineas Dominicanas S.A. -
Black List of the Air Operators
25th June 2015 LEGAL ANNOUNCEMENT The civil air transportation authorities of each member states of the European Union may check only the aircrafts of the airlines that provide flights to and from the airports of the European Union; and due to random nature of such checks, it is not possible to check each aircraft that lands at each European Union airport. The fact that the airline is not indicated on the list of the Association does not automatically mean that such company complies with the respective safety standards. If the company that is currently indicated on the list of the Association assumes that it complies with the necessary technical standards and requirements indicated in the respective international safety standards, it might request the Committee to initiate the proceedings to withdraw it from the list. All possible effort was made to verify the exact identity of all airlines indicated on the list of the Association – particularly with regard to the following properties: specific (and unique) letter codes for each airline by the ICAO organisation of the country that has issued the certificate as well as the certificate numbers (or the operating licence) of the air transport operator. Nevertheless, the complete verification was not in all cases possible due to the general lack of information on some airlines that could be borderline with the respected international system of the air transportation or entirely inconsistent with it. For this reason we cannot exclude the fact that there might be companies that conduct their business activities in good faith under the same business name as some of the airlines indicated in the list of the Association. -

Tijaris 126:Tijaris 107 11/07/04 23:47 Page 1
Tijaris 126:Tijaris 107 11/07/04 23:47 Page 1 TIJARIS Published by the Islamic Centre for Development of Trade issue 126 - June-August 2012 SPECIAL COUNTRY REPUBLIC OF GUINEA INTERVIEW WITH H.E Mrs. Baldé Hadja Mariama BAH, Minister of Hospitality, Tourism and Handicrafts of the Republic of Guinea 10 - 12 December 2012 Expo Centre Sharjah United Arab Emirates SPECIAL ISSUE International Food & Technology Exhibition THE WEST AFRICAN ECONOMIC AND MONETARY UNION (WAEMU) www.icdt-oic.org www.halalfoodme.com Tijaris 126:Tijaris 107 11/07/04 23:48 Page 2 IT IS AS SIMPLE AS THAT TRUST TIJARIS Since “Tijaris” is disseminated to 57 Member States and read by economic operators, take advantage of the business opportunities appearing on it to generate more trade flows. We want indeed to make of this magazine a suitable space for dialogue, firstly, for economic operators and secondly, to create unlimited opportunities to get your products well known... So entrust the advertisements of your products and services to us, trust “Tijaris” and be sure that your adverts will be widely disseminated by this magazine in such a manner as to meet market requirements... We are thus convinced that “Tijaris” is the most suitable advertising medium to make known your products and services by the economic operators of the Islamic World which constitutes a market of over 1 billion of consumers. So subscribe now and make your adverts on our magazine. To subscribe to “Tijaris” and use its advertising services, visit our website: www.icdt-oic.org or contact Mrs. Kadiatou -

EU) No 659/2013 of 10 July 2013 Amending Regulation (EC
L 190/54 EN Official Journal of the European Union 11.7.2013 COMMISSION IMPLEMENTING REGULATION (EU) No 659/2013 of 10 July 2013 amending Regulation (EC) No 474/2006 establishing the Community list of air carriers which are subject to an operating ban within the Community (Text with EEA relevance) THE EUROPEAN COMMISSION, the framework of Regulation (EC) No 2111/2005 and its implementing Regulation (EC) No 473/2006, with competent authorities and air carriers of the states of Having regard to the Treaty on the Functioning of the European Curaçao & St Maarten, Republic of Guinea, India, Iran, Union, Kazakhstan, Kyrgyzstan, Mozambique and Nepal. The Air Safety Committee also received updates from the Having regard to Regulation (EC) No 2111/2005 of the Commission about technical consultations with the European Parliament and the Council of 14 December 2005 Russian Federation and concerning monitoring of on the establishment of a Community list of air carriers subject Bolivia, Tajikistan and Turkmenistan. to an operating ban within the Community and on informing air passengers of the identity of the operating carrier, and repealing Article 9 of Directive 2004/36/CE ( 1 ), and in particular (6) The Air Safety Committee has heard presentations by Article 4 thereof ( 2), EASA about the results of the analysis of audit reports carried out by the International Civil Aviation Organi sation ('ICAO') in the framework of ICAO’s Universal Whereas: Safety Oversight Audit Programme ('USOAP'). Member States were invited to prioritize ramp inspections on (1) Commission Regulation (EC) No 474/2006 of 22 March air carriers licensed by states in respect of which 2006 ( 3 ) established the Community list of air carriers Significant Safety Concerns ('SSC') have been identified which are subject to an operating ban within the by ICAO or in respect of which EASA concluded that Union referred to in Chapter II of Regulation (EC) No there are significant deficiencies in the safety oversight 2111/2005. -

1. Airlines and Routes
Report No. 49194 Public Disclosure Authorized AFRICA INFRASTRUCTURE COUNTRY DIAGNOSTIC Air Transport Challenges to Growth Public Disclosure Authorized June 2009 Sustainable Development Africa Region Public Disclosure Authorized Document of the World Bank Public Disclosure Authorized Vice President: Obiageli Katryn Ezekwesili Sector Director: Inger Andersen Task Team Leader: Vivien Foster About AICD This study is part of the Africa Infrastructure Country Diagnostic (AICD), a project designed to expand the world’s knowledge of physical infrastructure in Africa. AICD will provide a baseline against which future improvements in infrastructure services can be measured, making it possible to monitor the results achieved from donor support. It should also provide a more solid empirical foundation for prioritizing investments and designing policy reforms in the infrastructure sectors in Africa. AICD will produce a series of reports (such as this one) that provide an overview of the status of public expenditure, investment needs, and sector performance in each of the main infrastructure sectors, including energy, information and communication technologies, irrigation, transport, and water and sanitation. The World Bank will publish a summary of AICD’s findings in spring 2008. The underlying data will be made available to the public, through an interactive Web site, allowing users to download customized data reports and perform simple simulation exercises. QuickTime™ and a TIFF (Uncompressed) decompressor are needed to see this picture. The first phase of AICD focuses on 24 countries that together account for 85 percent of the gross domestic product (GDP), population, and infrastructure aid flows of Sub-Saharan Africa. The countries are: Benin, Burkina Faso, Cape Verde, Cameroon, Chad, Congo (Democratic Republic of Congo), Côte d’Ivoire, Ethiopia, Ghana, Kenya, Madagascar, Malawi, Mali, Mozambique, Namibia, Niger, Nigeria, Rwanda, Senegal, South Africa, Sudan, Tanzania, Uganda, and Zambia. -

Annuaire Statistique Des Travaux Publics Et Des Transports
REPUBLIQUE DU BENIN __________ MINISTERE DES TRAVAUX PUBLICS ET DES MINISTERE DE L’ECONOMIE MARITIME TRANSPORTS ET DES INFRASTRUCTURES PORTUAIRES __________ DIRECTION DE LA PROGRAMMATION ET DE LA PROSPECTIVE __________ ANNUAIRE STATISTIQUE DES TRANSPORTS 2001-2008 N° 000 Cotonou, Mars 2014 REPUBLIQUE DU BENIN __________ MINISTERE DES TRAVAUX PUBLICS ET DES MINISTERE DE L’ECONOMIE MARITIME TRANSPORTS ET DES INFRASTRUCTURES PORTUAIRES __________ DIRECTION DE LA PROGRAMMATION ET DE LA PROSPECTIVE __________ ANNUAIRE DES STATISTIQUES DES TRANSPORTS 2001-2008 Equipe technique de rédaction - ABIALA Stanislas - CODJA Sourou - CHOUCHOU Obed - DJINKPO Médard - DJOGBENOU GODONOU Prosper - DJOSSA Laurent - HODONOU Assogba - KOUTON Désiré M. - ODOUBOUROU Ignace - TONAHIN Félix - VIGAN Fortuné Equipe technique de relecture - ABIKANLOU Yézid - KOUTON Narcisse - LOUPEDA Achille - SOHOTO Thierry - SONON D. Gustave - TAMBAMOU Géronimo - VLAVONOU YEMADJE Lucrèce Cotonou, Mars 2014 2 REMERCIEMENTS Le comité technique de rédaction remercie les différents acteurs internes et externes du secteur des transports qui ont contribué à l’élaboration du présent document. Convaincu que les utilisateurs y trouveront matière à analyse et réflexion, il sollicite d’ores et déjà leur indulgence et serait heureux de recevoir leurs critiques et suggestions pour une amélioration des prochaines éditions. 3 PHOTOS DES DIFFERENTS MINISTRES CHARGES DES TRANSPORTS DE 2001 A 2008 ATTIN Sourou Joseph AKOBI Ahamed Mme OMICHESSAN TABELE Christiane (15 Mai 1998 - 17 Juin 2003) (17 Juin 2003 –Février 2005) (Février 2005-8 Avril 2006) DOSSOU Kpèdéti Alexandre SENOU M. Richard ZINZINDOHOUE Armand (8 Avril 2006-19 Novembre 2006) (19 Novembre 2006-18 Juin 2007) (18 Juin 2007-23 Octobre 2008) 4 RESUME Le secteur des transports joue un rôle important dans la réduction de la pauvreté en tant que tel, il est important de fournir des informations nécessaires pouvant permettre une bonne prise de décision et une meilleure planification. -

Yamoussoukro Decision
Public Disclosure Authorized DIRECTIONS IN DEVELOPMENT Public Disclosure Authorized Infrastructure Open Skies for Africa Implementing the Yamoussoukro Decision Charles E. Schlumberger Public Disclosure Authorized Public Disclosure Authorized Open Skies for Africa Open Skies for Africa Implementing the Yamoussoukro Decision Charles E. Schlumberger © 2010 The International Bank for Reconstruction and Development / The World Bank 1818 H Street, NW Washington, DC 20433 Telephone 202-473-1000 Internet www.worldbank.org E-mail [email protected] All rights reserved. 1 2 3 4 :: 13 12 11 10 This volume is a product of the staff of the International Bank for Reconstruction and Development / The World Bank. The findings, interpretations, and conclusions expressed in this volume do not necessarily reflect the views of the Executive Directors of The World Bank or the governments they represent. The World Bank does not guarantee the accuracy of the data included in this work. The bound- aries, colors, denominations, and other information shown on any map in this work do not imply any judgment on the part of The World Bank concerning the legal status of any territory or the endorsement or acceptance of such boundaries. Rights and Permissions The material in this publication is copyrighted. Copying and/or transmitting portions or all of this work without permission may be a violation of applicable law. The International Bank for Reconstruction and Development / The World Bank encourages dissemination of its work and will normally grant permission to reproduce portions of the work promptly. For permission to photocopy or reprint any part of this work, please send a request with complete information to the Copyright Clearance Center Inc., 222 Rosewood Drive, Danvers, MA 01923, USA; telephone: 978-750-8400; fax: 978-750-4470; Internet: www.copyright.com. -

World Bank Document
Document of The World Bank FOR OFFICIAL USE ONLY Public Disclosure Authorized Report No: 44008-AFR PROJECT APPRAISAL DOCUMENT ON A PROPOSED GRANT TO THE REPUBLIC OF BENIN IN THE AMOUNT OF SDR 6.1 MILLION (US$9 MILLION EQUIVALENT) AND Public Disclosure Authorized ON A PROPOSED CREDIT TO THE REPUBLIC OF SENEGAL IN THE AMOUNT OF SDR 4.70 MILLION (US$7 MILLION EQUIVALENT) IN SUPPORT OF SECOND PHASE “B” OF WEST AND CENTRAL AFRICA AIR TRANSPORT SAFETY & SECURITY ADAPTABLE PROGRAM LENDNG (APL) PROGRAM Public Disclosure Authorized February 3,2009 Africa Transport Sector Country Department AFRCI Africa Regional Office This document has a restricted distribution and may be used by Recipients only in the performance of their official duties. Its contents may not otherwise be disclosed without World Bank authorization. Public Disclosure Authorized CURRENCY EQUIVALENTS (Exchange Rate Effective October 3 1, 2008) CurrencyUnit = FCFA FCFA 51 1 US$l US$1.49 = SDR 1 FISCAL YEAR January 1 - December31 ABBREVIATIONS AND ACRONYMS AAANS Administration of Senegal National Air Activities ADB African Development Bank AFTN Aeronautical Fixed Telecommunication Network AMHS Aeronautical Message Handling System ANAC Agence Nationale de 1 'Aviation Civile (National Civil Aviation Agency) APL Adaptable Program Lending ARMP Autorite' de Re'gulation des Marche's Publics (Public Procurement Regulatory Authority) ASECNA Agence pour la Se'curite' de la Navigation Ae'rienne en Afrique et a Madagascar (Agency for Air Navigation Safety in Africa and Madagascar) ATM Air Traffic