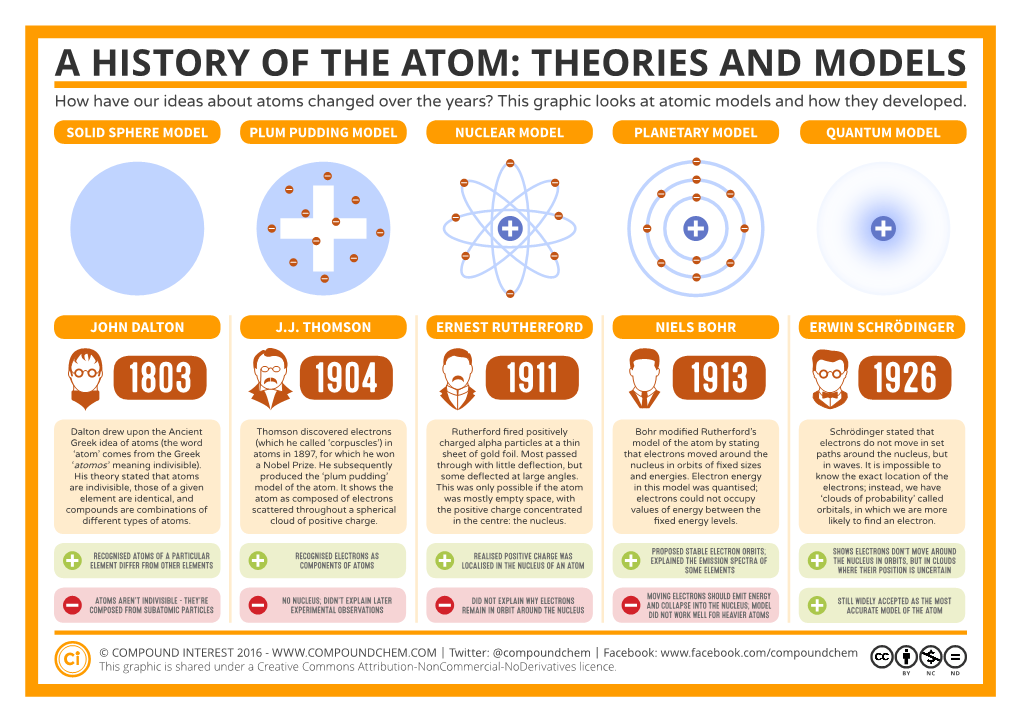
A HISTORY of the ATOM: THEORIES and MODELS How Have Our Ideas About Atoms Changed Over the Years? This Graphic Looks at Atomic Models and How They Developed
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Neutron Stars
Chandra X-Ray Observatory X-Ray Astronomy Field Guide Neutron Stars Ordinary matter, or the stuff we and everything around us is made of, consists largely of empty space. Even a rock is mostly empty space. This is because matter is made of atoms. An atom is a cloud of electrons orbiting around a nucleus composed of protons and neutrons. The nucleus contains more than 99.9 percent of the mass of an atom, yet it has a diameter of only 1/100,000 that of the electron cloud. The electrons themselves take up little space, but the pattern of their orbit defines the size of the atom, which is therefore 99.9999999999999% Chandra Image of Vela Pulsar open space! (NASA/PSU/G.Pavlov et al. What we perceive as painfully solid when we bump against a rock is really a hurly-burly of electrons moving through empty space so fast that we can't see—or feel—the emptiness. What would matter look like if it weren't empty, if we could crush the electron cloud down to the size of the nucleus? Suppose we could generate a force strong enough to crush all the emptiness out of a rock roughly the size of a football stadium. The rock would be squeezed down to the size of a grain of sand and would still weigh 4 million tons! Such extreme forces occur in nature when the central part of a massive star collapses to form a neutron star. The atoms are crushed completely, and the electrons are jammed inside the protons to form a star composed almost entirely of neutrons. -

TEK 8.5C: Periodic Table
Name: Teacher: Pd. Date: TEK 8.5C: Periodic Table TEK 8.5C: Interpret the arrangement of the Periodic Table, including groups and periods, to explain how properties are used to classify elements. Elements and the Periodic Table An element is a substance that cannot be separated into simpler substances by physical or chemical means. An element is already in its simplest form. The smallest piece of an element that still has the properties of that element is called an atom. An element is a pure substance, containing only one kind of atom. The Periodic Table of Elements is a list of all the elements that have been discovered and named, with each element listed in its own element square. Elements are represented on the Periodic Table by a one or two letter symbol, and its name, atomic number and atomic mass. The Periodic Table & Atomic Structure The elements are listed on the Periodic Table in atomic number order, starting at the upper left corner and then moving from the left to right and top to bottom, just as the words of a paragraph are read. The element’s atomic number is based on the number of protons in each atom of that element. In electrically neutral atoms, the atomic number also represents the number of electrons in each atom of that element. For example, the atomic number for neon (Ne) is 10, which means that each atom of neon has 10 protons and 10 electrons. Magnesium (Mg) has an atomic number of 12, which means it has 12 protons and 12 electrons. -

Centripetal Force Is Balanced by the Circular Motion of the Elctron Causing the Centrifugal Force
STANDARD SC1 b. Construct an argument to support the claim that the proton (and not the neutron or electron) defines the element’s identity. c. Construct an explanation based on scientific evidence of the production of elements heavier than hydrogen by nuclear fusion. d. Construct an explanation that relates the relative abundance of isotopes of a particular element to the atomic mass of the element. First, we quickly review pre-requisite concepts One of the most curious observations with atoms is the fact that there are charged particles inside the atom and there is also constant spinning and Warm-up 1: List the name, charge, mass, and location of the three subatomic circling. How does atom remain stable under these conditions? Remember particles Opposite charges attract each other; Like charges repel each other. Your Particle Location Charge Mass in a.m.u. Task: Read the following information and consult with your teacher as STABILITY OF ATOMS needed, answer Warm-Up tasks 2 and 3 on Page 2. (3) Death spiral does not occur at all! This is because the centripetal force is balanced by the circular motion of the elctron causing the centrifugal force. The centrifugal force is the outward force from the center to the circumference of the circle. Electrons not only spin on their own axis, they are also in a constant circular motion around the nucleus. Despite this terrific movement, electrons are very stable. The stability of electrons mainly comes from the electrostatic forces of attraction between the nucleus and the electrons. The electrostatic forces are also known as Coulombic Forces of Attraction. -

Of the Periodic Table
of the Periodic Table teacher notes Give your students a visual introduction to the families of the periodic table! This product includes eight mini- posters, one for each of the element families on the main group of the periodic table: Alkali Metals, Alkaline Earth Metals, Boron/Aluminum Group (Icosagens), Carbon Group (Crystallogens), Nitrogen Group (Pnictogens), Oxygen Group (Chalcogens), Halogens, and Noble Gases. The mini-posters give overview information about the family as well as a visual of where on the periodic table the family is located and a diagram of an atom of that family highlighting the number of valence electrons. Also included is the student packet, which is broken into the eight families and asks for specific information that students will find on the mini-posters. The students are also directed to color each family with a specific color on the blank graphic organizer at the end of their packet and they go to the fantastic interactive table at www.periodictable.com to learn even more about the elements in each family. Furthermore, there is a section for students to conduct their own research on the element of hydrogen, which does not belong to a family. When I use this activity, I print two of each mini-poster in color (pages 8 through 15 of this file), laminate them, and lay them on a big table. I have students work in partners to read about each family, one at a time, and complete that section of the student packet (pages 16 through 21 of this file). When they finish, they bring the mini-poster back to the table for another group to use. -

Pioneers of Atomic Theory Darius Bermudez Discoverers of the Atom
Pioneers of Atomic Theory Darius Bermudez Discoverers of the Atom Democritus- Greek Philosopher proposed that if something was divided enough times, eventually the particles would be too small to divide any further. Ex: Identify this Greek philosopher who postulated that if an object was divided enough times, there would eventually be small particles that could not be divided any further. Discoverers of the Atom John Dalton- English chemist who made the “billiard ball” atom model. First to prove that rainfall was a result of temperature change. He was the first scientist after Democritus to build on atomic theory. He also created a law on partial pressures. Common Clues: Partial pressures, pioneer of atomic theory, and temperature change causes rainfall. The image cannot be displayed. Your computer may not have enough memory to open the image, or the image may have been corrupted. Restart your computer, and then open the file again. If the red x still appears, you may have to delete the image and then insert it again. The image cannot be displayed. Your computer may not have enough memory to open the image, or the image may have been corrupted. Restart your computer, and then open the file again. If the red x still appears, you may have to delete the image and then insert it again. Discoverers of the Atom J.J. Thomson- English Scientist who discovered electrons through a cathode. Made the “plum pudding model” with Lord Kelvin (Kelvin Scale) which stated that negative charges were spread about a positive charged medium, making atoms neutral. Common Clues: Plum pudding, Electrons had negative charges, disproved by either Rutherford or Mardsen and Geiger The image cannot be displayed. -

Introduction to Chemistry
Introduction to Chemistry Author: Tracy Poulsen Digital Proofer Supported by CK-12 Foundation CK-12 Foundation is a non-profit organization with a mission to reduce the cost of textbook Introduction to Chem... materials for the K-12 market both in the U.S. and worldwide. Using an open-content, web-based Authored by Tracy Poulsen collaborative model termed the “FlexBook,” CK-12 intends to pioneer the generation and 8.5" x 11.0" (21.59 x 27.94 cm) distribution of high-quality educational content that will serve both as core text as well as provide Black & White on White paper an adaptive environment for learning. 250 pages ISBN-13: 9781478298601 Copyright © 2010, CK-12 Foundation, www.ck12.org ISBN-10: 147829860X Except as otherwise noted, all CK-12 Content (including CK-12 Curriculum Material) is made Please carefully review your Digital Proof download for formatting, available to Users in accordance with the Creative Commons Attribution/Non-Commercial/Share grammar, and design issues that may need to be corrected. Alike 3.0 Unported (CC-by-NC-SA) License (http://creativecommons.org/licenses/by-nc- sa/3.0/), as amended and updated by Creative Commons from time to time (the “CC License”), We recommend that you review your book three times, with each time focusing on a different aspect. which is incorporated herein by this reference. Specific details can be found at http://about.ck12.org/terms. Check the format, including headers, footers, page 1 numbers, spacing, table of contents, and index. 2 Review any images or graphics and captions if applicable. -

Elements Make up the Periodic Table
Page 1 of 7 KEY CONCEPT Elements make up the periodic table. BEFORE, you learned NOW, you will learn • Atoms have a structure • How the periodic table is • Every element is made from organized a different type of atom • How properties of elements are shown by the periodic table VOCABULARY EXPLORE Similarities and Differences of Objects atomic mass p. 17 How can different objects be organized? periodic table p. 18 group p. 22 PROCEDURE MATERIALS period p. 22 buttons 1 With several classmates, organize the buttons into three or more groups. 2 Compare your team’s organization of the buttons with another team’s organization. WHAT DO YOU THINK? • What characteristics did you use to organize the buttons? • In what other ways could you have organized the buttons? Elements can be organized by similarities. One way of organizing elements is by the masses of their atoms. Finding the masses of atoms was a difficult task for the chemists of the past. They could not place an atom on a pan balance. All they could do was find the mass of a very large number of atoms of a certain element and then infer the mass of a single one of them. Remember that not all the atoms of an element have the same atomic mass number. Elements have isotopes. When chemists attempt to measure the mass of an atom, therefore, they are actually finding the average mass of all its isotopes. The atomic mass of the atoms of an element is the average mass of all the element’s isotopes. -

Lecture #3, Atomic Structure (Rutherford, Bohr Models)
Welcome to 3.091 Lecture 3 September 14, 2009 Atomic Models: Rutherford & Bohr Periodic Table Quiz 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 31 32 33 34 35 36 37 38 39 40 41 42 43 44 45 46 47 48 49 50 51 52 53 54 55 56 57 72 73 74 75 76 77 78 79 80 81 82 83 84 85 86 87 88 89 Name Grade /10 Image by MIT OpenCourseWare. La Lazy Ce college Pr professors Nd never Pm produce Sm sufficiently Eu educated Gd graduates Tb to Dy dramatically Ho help Er executives Tm trim Yb yearly Lu losses. © source unknown. All rights reserved. This image is excluded from our Creative Commons license. For more information, see http://ocw.mit.edu/fairuse. La Loony Ce chemistry Pr professor Nd needs Pm partner: Sm seeking cannot be referring Eu educated to 3.091! Gd graduate Tb to must be the “other” Dy develop Ho hazardous chemistry professor Er experiments Tm testing Yb young Lu lab assistants. © source unknown. All rights reserved. This image is excluded from our Creative Commons license. For more information, see http://ocw.mit.edu/fairuse. 138.9055 920 57 3455 3 6.146 * 1.10 57 5.577 La [Xe]5d16s2 Lanthanum CEase not I to slave, back breaking to tend; PRideless and bootless stoking hearth and fire. No Dream of mine own precious time to spend Pour'ed More to sate your glutt'nous desire. -

Electron Charge Density: a Clue from Quantum Chemistry for Quantum Foundations
Electron Charge Density: A Clue from Quantum Chemistry for Quantum Foundations Charles T. Sebens California Institute of Technology arXiv v.2 June 24, 2021 Forthcoming in Foundations of Physics Abstract Within quantum chemistry, the electron clouds that surround nuclei in atoms and molecules are sometimes treated as clouds of probability and sometimes as clouds of charge. These two roles, tracing back to Schr¨odingerand Born, are in tension with one another but are not incompatible. Schr¨odinger'sidea that the nucleus of an atom is surrounded by a spread-out electron charge density is supported by a variety of evidence from quantum chemistry, including two methods that are used to determine atomic and molecular structure: the Hartree-Fock method and density functional theory. Taking this evidence as a clue to the foundations of quantum physics, Schr¨odinger'selectron charge density can be incorporated into many different interpretations of quantum mechanics (and extensions of such interpretations to quantum field theory). Contents 1 Introduction2 2 Probability Density and Charge Density3 3 Charge Density in Quantum Chemistry9 3.1 The Hartree-Fock Method . 10 arXiv:2105.11988v2 [quant-ph] 24 Jun 2021 3.2 Density Functional Theory . 20 3.3 Further Evidence . 25 4 Charge Density in Quantum Foundations 26 4.1 GRW Theory . 26 4.2 The Many-Worlds Interpretation . 29 4.3 Bohmian Mechanics and Other Particle Interpretations . 31 4.4 Quantum Field Theory . 33 5 Conclusion 35 1 1 Introduction Despite the massive progress that has been made in physics, the composition of the atom remains unsettled. J. J. Thomson [1] famously advocated a \plum pudding" model where electrons are seen as tiny negative charges inside a sphere of uniformly distributed positive charge (like the raisins|once called \plums"|suspended in a plum pudding). -

Cbiescss05.Pdf
Science IX Sample Paper 5 Solved www.rava.org.in CLASS IX (2019-20) SCIENCE (CODE 086) SAMPLE PAPER-5 Time : 3 Hours Maximum Marks : 80 General Instructions : (i) The question paper comprises of three sections-A, B and C. Attempt all the sections. (ii) All questions are compulsory. (iii) Internal choice is given in each sections. (iv) All questions in Section A are one-mark questions comprising MCQ, VSA type and assertion-reason type questions. They are to be answered in one word or in one sentence. (v) All questions in Section B are three-mark, short-answer type questions. These are to be answered in about 50-60 words each. (vi) All questions in Section C are five-mark, long-answer type questions. These are to be answered in about 80-90 words each. (vii) This question paper consists of a total of 30 questions. 4. What is the S.I. unit of momentum ? [1] SECTION -A (a) kgms (b) mskg−1 −1 −1 DIRECTION : For question numbers 1 and 2, two statements (c) kgms (d) kg() ms are given- one labelled Assertion (A) and the other labelled Ans : (c) kgms−1 Reason (R). Select the correct answer to these questions from the codes (a), (b), (c) and (d) as given below. 5. Which of the following is not a perfectly in elastic collision ? [1] (a) Both A and R are true and R is correct explanation (a) Capture of an electron by proton. of the assertion. (b) Man jumping on to a moving cart. (b) Both A and R are true but R is not the correct explanation of the assertion. -

Make an Atom Vocabulary Grade Levels
MAKE AN ATOM Fundamental to physical science is a basic understanding of the atom. Atoms are comprised of protons, neutrons, and electrons. Protons and neutrons are at the center of the atom while electrons live in lobe-shaped clouds outside the nucleus. The number of electrons usually matches the number of protons, yielding a net neutral charge for the atom. Sometimes an atom has less neutrons or more neutrons than protons. This is called an isotope. If an atom has different numbers of electrons than protons, then it is an ion. If an atom has different numbers of protons, it is a different element all together. Scientists at Idaho National Laboratory study, create, and use radioactive isotopes like Uranium 234. The 234 means this isotope has an atomic mass of 234 Atomic Mass Units (AMU). GRADE LEVELS: 3-8 VOCABULARY Atom – The basic unit of a chemical element. Proton – A stable subatomic particle occurring in all atomic nuclei, with a positive electric charge equal in magnitude to that of an electron, but of opposite sign. Neutron – A subatomic particle of about the same mass as a proton but without an electric charge, present in all atomic nuclei except those of ordinary hydrogen. Electron – A stable subatomic particle with a charge of negative electricity, found in all atoms and acting as the primary carrier of electricity in solids. Orbital – Each of the actual or potential patterns of electron density that may be formed on an atom or molecule by one or more electrons. Ion – An atom or molecule with a net electric charge due to the loss or gain of one or more electrons. -

Coercing Magnetism Into Diamagnetic Ceramics: a Case Study in Alumina Erik Nykwest University of Connecticut - Storrs, [email protected]
University of Connecticut Masthead Logo OpenCommons@UConn Doctoral Dissertations University of Connecticut Graduate School 4-25-2019 Coercing Magnetism into Diamagnetic Ceramics: A Case Study in Alumina Erik Nykwest University of Connecticut - Storrs, [email protected] Follow this and additional works at: https://opencommons.uconn.edu/dissertations Recommended Citation Nykwest, Erik, "Coercing Magnetism into Diamagnetic Ceramics: A Case Study in Alumina" (2019). Doctoral Dissertations. 2136. https://opencommons.uconn.edu/dissertations/2136 Coercing Magnetism into Diamagnetic Ceramics: A Case Study in Alumina Erik Carl Nykwest, Ph.D. University of Connecticut, 2019 Ceramics are very diverse class of materials whose properties can vary greatly. It is this diversity that make ceramics so useful in advanced technology. The relatively open crystal structure of ceramics makes it pos- sible to impart functionalities via judicious doping. This work focuses on developing a generalized method for introducing magnetism into normally non-magnetic (diamagnetic) ceramics, using the example case of alumina (Al2O3).Here, substitutional doping of Al atoms with 3d transition metal in α- and θ-alumina was studied. Density functional theory was used to predict the structural, electronic, and magnetic properties of doped alumina, as well as its stability. The results show that adding small concentrations of transition metals to alumina may increase magnetic activity by generating unpaired electrons whose net magnetic moments may couple with external magnetic fields. The dopant species and dopant coordination environment are the most important factors in determining the spin density distribution (localized or delocalized from the dopant atom) and net magnetic moment, which strongly direct the ability of the doped alumina to couple with an ex- ternal field.