FAUNA and FLORA of the BAY of NAPLES
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-
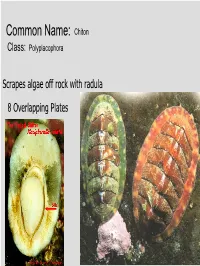
Common Name: Chiton Class: Polyplacophora
Common Name: Chiton Class: Polyplacophora Scrapes algae off rock with radula 8 Overlapping Plates Phylum? Mollusca Class? Gastropoda Common name? Brown sea hare Class? Scaphopoda Common name? Tooth shell or tusk shell Mud Tentacle Foot Class? Gastropoda Common name? Limpet Phylum? Mollusca Class? Bivalvia Class? Gastropoda Common name? Brown sea hare Phylum? Mollusca Class? Gastropoda Common name? Nudibranch Class? Cephalopoda Cuttlefish Octopus Squid Nautilus Phylum? Mollusca Class? Gastropoda Most Bivalves are Filter Feeders A B E D C • A: Mantle • B: Gill • C: Mantle • D: Foot • E: Posterior adductor muscle I.D. Green: Foot I.D. Red Gills Three Body Regions 1. Head – Foot 2. Visceral Mass 3. Mantle A B C D • A: Radula • B: Mantle • C: Mouth • D: Foot What are these? Snail Radulas Dorsal HingeA Growth line UmboB (Anterior) Ventral ByssalC threads Mussel – View of Outer Shell • A: Hinge • B: Umbo • C: Byssal threads Internal Anatomy of the Bay Mussel A B C D • A: Labial palps • B: Mantle • C: Foot • D: Byssal threads NacreousB layer Posterior adductorC PeriostracumA muscle SiphonD Mantle Byssal threads E Internal Anatomy of the Bay Mussel • A: Periostracum • B: Nacreous layer • C: Posterior adductor muscle • D: Siphon • E: Mantle Byssal gland Mantle Gill Foot Labial palp Mantle Byssal threads Gill Byssal gland Mantle Foot Incurrent siphon Byssal Labial palp threads C D B A E • A: Foot • B: Gills • C: Posterior adductor muscle • D: Excurrent siphon • E: Incurrent siphon Heart G F H E D A B C • A: Foot • B: Gills • C: Mantle • D: Excurrent siphon • E: Incurrent siphon • F: Posterior adductor muscle • G: Labial palps • H: Anterior adductor muscle Siphon or 1. -

First Three-Dimensionally Preserved in Situ Record of an Aptychophoran Ammonite Jaw Apparatus in the Jurassic and Discussion of the Function of Aptychi
Berliner paläobiologische Abhandlungen 10 321-330 Berlin 2009-11-11 First three-dimensionally preserved in situ record of an aptychophoran ammonite jaw apparatus in the Jurassic and discussion of the function of aptychi Günter Schweigert Abstract: A unique specimen of the microconch ammonite Lingulaticeras planulatum Berckhemer in Ziegler, 1958 comes from a tempestite bed within the Upper Jurassic lithographic limestones of Scham- haupten in Franconia (Painten Formation, uppermost Kimmeridgian). The shell is unique because it retains the complete jaw apparatus in the body chamber. The articulation of the Lamellaptychus and the corresponding upper beak are well preserved. The function of the aptychus is discussed in general, and an operculum function is thought to be unlikely. The formation of strongly calcified aptychi in aspidoceratids and some oppeliid ammonoids is interpreted as an added ballast weight to stabilize the conch for swimming in the water column. Keywords: Ammonites, aptychus, preservation, functional morphology, Upper Jurassic, lithographic lime- stones, Franconia, Germany Zusammenfassung: Ein einzigartig erhaltenes Exemplar des mikroconchen Ammoniten Lingulaticeras planulatum Berckhemer in Ziegler, 1958 aus einer Tempestitlage des oberjurassischen Plattenkalks von Schamhaupten in Franken (Painten-Formation, oberstes Kimmeridgium) enthält noch den vollständigen Kieferapparat in seiner Wohnkammer.Es zeigt die perfekte Artikualation des Lamellaptychus mit dem dazu- gehörenden Oberkiefer. Die Funktion des Aptychus wird allgemein diskutiert und eine Deckelfunktion für unwahrscheinlich gehalten. Die Ausbildung stark verkalkter Aptychen wie in Aspidoceraten und manchen Oppeliiden wird als zusätzliches Tariergewicht gedeutet, um das Gehäuse in starker bewegtem Wasser zu stabilisieren. Schlüsselwörter: Ammoniten, Aptychus, Erhaltung, Plattenkalke, Funktionsmorphologie, Oberjura, Franken, Deutschland Address of the author: Dr. Günter Schweigert, Staatliches Museum für Naturkunde, Rosenstein 1, D-70191 Stuttgart. -

In Bahia, Brazil
Volume 52(40):515‑524, 2012 A NEW GENUS AND SPECIES OF CAVERNICOLOUS POMATIOPSIDAE (MOLLUSCA, CAENOGASTROPODA) IN BAHIA, BRAZIL 1 LUIZ RICARDO L. SIMONE ABSTRACT Spiripockia punctata is a new genus and species of Pomatiopsidae found in a cave from Serra Ramalho, SW Bahia, Brazil. The taxon is troglobiont (restricted to subterranean realm), and is characterized by the shell weakly elongated, fragile, translucent, normally sculptured by pus‑ tules with periostracum hair on tip of pustules; peristome highly expanded; umbilicus opened; radular rachidian with 6 apical and 3 pairs of lateral cusps; osphradium short, arched; gill filaments with rounded tip; prostate flattened, with vas deferens inserting subterminally; penis duct narrow and weakly sinuous; pallial oviduct simple anteriorly, possessing convoluted by‑ pass connecting base of bulged portion of transition between visceral and pallial oviducts with base of seminal receptacle; spermathecal duct complete, originated from albumen gland. The description of this endemic species may raise protective environmental actions to that cave and to the Serra Ramalho Karst area. Key-Words: Pomatiopsidae; Spiripockia punctata gen. nov. et sp. nov.; Brazil; Cave; Tro- globiont; Anatomy. INTRODUCTION An enigmatic tiny gastropod has been collected in caves from the Serra Ramalho Kars area, southwestern The family Pomatiopsidae is represented in the Bahia state, Brazil. It has a pretty, fragile, translucent Brazilian region by only two species of the genus Id‑ shell in such preliminary gross anatomy, which already iopyrgus Pilsbry, 1911 (Simone, 2006: 94). However, reveals troglobiont adaptations, i.e., depigmentation, the taxon is much richer in remaining mainland ar- lack of eyes and small size. The sample has been brought eas, with both freshwater and semi-terrestrial habits by Maria Elina Bichuette, who is specialized in subter- (Ponder & Keyzer, 1998; Kameda & Kato, 2011). -

New Data on the Jaw Apparatus of Fossil Cephalopods
New dataon the jaw apparatus of fossil cephalopods YURI D. ZAKHAROV AND TAMAZ A. LOMINADZE \ Zakharov, Yuri D. & Lominadze, Tamaz A. 19830115: New data on the jaw apparatus of fossil LETHAIA cephalopods. Lethaia, Vol. 16, pp. 67-78. Oslo. ISSN 0024-1164. A newly discovered fossil cephalopod jaw apparatus that may belong to Permian representatives of the Endocochlia is described. Permorhynchus dentatus n. gen. n. sp. is established on the basis of this ~ apparatus. The asymmetry of jaws in the Ectocochlia may be connected with the double function of the ventral jaw apparatus, and the well-developed, relatively large frontal plate of the ventral jaw should be regarded as a feature common to all representatives of ectocochlian cephalopods evolved from early Palaeozoic stock. Distinct features seen in the jaw apparatus of Upper Pcrmian cndocochlians include the pronounced beak form of both jaws and the presence of oblong wings on the ventral mandible. o Cephalopoda. jaw. operculum. aptychus, anaptychus, Permorhynchus n.gen.• evolution. Permian. Yuri D. Zakharov llOpllll Ilscumpueeu« Gaxapoe], Institute of Biology and Pedology, Far-Eastern Scientific Centre. USSR Academy of Science, Vladivostok 690022, USSR (EUOJl020-n9~BnlHbliiuucmu my m Ilaot.neeocmo-cnoro Ha."~H020 uenmpav Axaoeuuu 'HayK CCCP, Bnaoueocmo« 690022, CCCP); Tamaz A. Lominadze ITa.'W3 Apl.j1L10BUl.j Jlouunaoee), Institute of Palaeobiology of Georgian SSR Academy of Science. Tbilisi 380004. USSR (Hncmumvm naJle06UOJl02UU Atcaoeuuu naytc TpY3UHUjKOii CCP. T6'LJUCU 380004. CCP; 19th August. 1980 (revised 1982 06 28). The jaw apparatus of Recent cephalopods is re Turek 1978, Fig. 7, but not the reconstruction in presented by two jaw elements (Fig. -

Copyrighted Material
319 Index a oral cavity 195 guanocytes 228, 231, 233 accessory sex glands 125, 316 parasites 210–11 heart 235 acidophils 209, 254 pharynx 195, 197 hemocytes 236 acinar glands 304 podocytes 203–4 hemolymph 234–5, 236 acontia 68 pseudohearts 206, 208 immune system 236 air sacs 305 reproductive system 186, 214–17 life expectancy 222 alimentary canal see digestive setae 191–2 Malpighian tubules 232, 233 system taxonomy 185 musculoskeletal system amoebocytes testis 214 226–9 Cnidaria 70, 77 typhlosole 203 nephrocytes 233 Porifera 28 antennae nervous system 237–8 ampullae 10 Decapoda 278 ocelli 240 Annelida 185–218 Insecta 301, 315 oral cavity 230 blood vessels 206–8 Myriapoda 264, 275 ovary 238 body wall 189–94 aphodus 38 pedipalps 222–3 calciferous glands 197–200 apodemes 285 pharynx 230 ciliated funnel 204–5 apophallation 87–8 reproductive system 238–40 circulatory system 205–8 apopylar cell 26 respiratory system 236–7 clitellum 192–4 apopyle 38 silk glands 226, 242–3 coelomocytes 208–10 aquiferous system 21–2, 33–8 stercoral sac 231 crop 200–1 Arachnida 221–43 sucking stomach 230 cuticle 189 biomedical applications 222 taxonomy 221 diet 186–7 body wall 226–9 testis 239–40 digestive system 194–203 book lungs 236–7 tracheal tube system 237 dissection 187–9 brain 237 traded species 222 epidermis 189–91 chelicera 222, 229 venom gland 241–2 esophagus 197–200 circulatory system 234–6 walking legs 223 excretory system 203–5 COPYRIGHTEDconnective tissue 228–9 MATERIALzoonosis 222 ganglia 211–13 coxal glands 232, 233–4 archaeocytes 28–9 giant nerve -

Biology and Description of Antisabia Juliae Sp. Nov., New Hipponicid Gastropod Commensal on Turbo Spp
SCI. MAR., 61 (Supl. 2): 5-14 SCIENTIA MARINA 1997 ECOLOGY OF MARINE MOLLUSCS. J.D. ROS and A. GUERRA (eds.) Biology and description of Antisabia juliae sp. nov., new Hipponicid gastropod commensal on Turbo spp. in Laing Island (Papua New Guinea)* MATHIEU POULICEK1, JEAN-CLAUDE BUSSERS1 and PIERRE VANDEWALLE2 1Animal Ecology Laboratory and 2Functional Morphology Laboratory, Zoological Institute, Liège University. 22, Quai Van Beneden, B-4020 Liège. Belgium. SUMMARY: The gastropod family Hipponicidae comprises widely distributed but poorly known sedentary species. On the beach-rock of the coral reefs of Laing Island (Papua New Guinea) live rich populations of several gastropod Turbo species of which many specimens have attached to their shell a hipponicid gastropod attributed to a new species, Antisabia juliae. This new species, described in this paper, appears to have adapted its mode of life on live turbinids in several ways result- ing in morphological changes (thin basal plate loosely adherent to the supporting shell, functional eyes, very long snout, functional radula, small osphradium) and ethological changes (foraging behaviour: it appears to feed on the epiphytic com- munity growing on the host, in the vicinity of the “host” shell). Except for these characteristics, the mode of life appears quite similar to that of other hipponicid species with few big females surrounded by several much smaller males. Development occurs within the egg mass inside the female shell and a few young snails escape at the crawling stage. Key words: Mollusca, Gastropoda, ecology, Hipponicidae, Papua New Guinea, Indopacific. RESUMEN: BIOLOGÍA Y DESCRIPCIÓN DE ANTISABIA JULIAE SP. NOV., UN NUEVO GASTERÓPODO HIPONÍCIDO COMENSAL DE TURBO SPP. -

Octopus Insularis</Italic> As a New Marine Model for Evolutionary
© 2019. Published by The Company of Biologists Ltd | Biology Open (2019) 8, bio046086. doi:10.1242/bio.046086 RESEARCH ARTICLE Octopus insularis as a new marine model for evolutionary developmental biology Ernesto Maldonado1,*, Emma Rangel-Huerta1,2, Roberto González-Gómez3,4, Gabriel Fajardo-Alvarado3,4 and Piedad S. Morillo-Velarde4,5,* ABSTRACT of aquatic animal eggs and embryos guarantees the observation of Octopuses are intriguing organisms that, together with squids and every developmental stage using microscopy and allows detailed cuttlefishes, form the extant coleoid cephalopods. This group includes experimental analysis from the first cell division through to the many species that can potentially be used as models in the fields of formation of embryonic germ layers and organogenesis (Boletzky biomedicine, developmental biology, evolution, neuroscience and et al., 2006). Finally, small embryos allow reasonable sample sizes even for robotics research. The purpose of this work is to first to be tested together using multi-well plates to provide multiple present a simple method for maintaining Octopus insularis embryos experimental replicates at the same time, making them cost- under a laboratory setup. Second, we show that these embryos are effective animal models (Hill et al., 2005). suitable for detailed analyses of specific traits that appear during Coleoid cephalopods (octopus, squid and cuttlefish) exhibit the developmental stages, including the eyes, hearts, arms, suckers, largest nervous systems found among invertebrates (Young, 1971) chromatophores and Kölliker’s organs. Similar complex traits between and a sophisticated visual system controlling body color changes for cephalopods and vertebrates such as the visual, cardiovascular, communication, camouflage and mimicry (Hanlon et al., 2011; neural and pigmentation systems are generally considered to be a Robin et al., 2014). -

Evolution of Nervous Systems and Brains 2
Evolution of Nervous Systems and Brains 2 Gerhard Roth and Ursula Dicke The modern theory of biological evolution, as estab- drift”) is incomplete; they point to a number of other lished by Charles Darwin and Alfred Russel Wallace and perhaps equally important mechanisms such as in the middle of the nineteenth century, is based on (i) neutral gene evolution without natural selection, three interrelated facts: (i) phylogeny – the common (ii) mass extinctions wiping out up to 90 % of existing history of organisms on earth stretching back over 3.5 species (such as the Cambrian, Devonian, Permian, and billion years, (ii) evolution in a narrow sense – Cretaceous-Tertiary mass extinctions) and (iii) genetic modi fi cations of organisms during phylogeny and and epigenetic-developmental (“ evo - devo ”) self-canal- underlying mechanisms, and (iii) speciation – the ization of evolutionary processes [ 2 ] . It remains uncer- process by which new species arise during phylogeny. tain as to which of these possible processes principally Regarding the phylogeny, it is now commonly accepted drive the evolution of nervous systems and brains. that all organisms on Earth are derived from a com- mon ancestor or an ancestral gene pool, while contro- versies have remained since the time of Darwin and 2.1 Reconstruction of the Evolution Wallace about the major mechanisms underlying the of Nervous Systems and Brains observed modi fi cations during phylogeny (cf . [1 ] ). The prevalent view of neodarwinism (or better In most cases, the reconstruction of the evolution of “new” or “modern evolutionary synthesis”) is charac- nervous systems and brains cannot be based on fossil- terized by the assumption that evolutionary changes ized material, since their soft tissues decompose, but are caused by a combination of two major processes, has to make use of the distribution of neural traits in (i) heritable variation of individual genomes within a extant species. -

Proceedings of the Academy of Natural Sciences of Philadelphia
376 PROCEEDINGS OF THE ACADEMY OF [May, A NEW SPECIES OF ONCHIDIOPSIS FROM BERING SEA. BY WILLIAM H. DALL. The 'genus Onchidiopsis Bergh (1853) was proposed for certain Arctic mollusks related to Velutina and possessing an internal nearly laminar shell. The minor characters of the few species known are in many respects different but their combinations are so intermixed that it is difficult to assign to the differences more than specific value. However the peculiarities of the present species are such that I venture to separate the genus into two sections, as follows : Genus ONCHIDIOPSIS Bergh, 1S53. Section ONCHIDIOPSIS, type 0. gronlandica Bergh. Adult animal with an impervious notseum. Section ATLANTOLIMAX, type 0. (A.) hannai Dall. Adult with a large dorsal foramen in the notseum. Onchidiopsis <Atlantolimax> hannai n. sp. Animal, after preservation in spirits, of a yellowish white color except on the sides of the foot and on the osphradium. The foot is muscular, broad, tapering and bluntly pointed behind, extending about one-third of its length behind the hinder margin of the no- tseum even when contracted; the front edge duplex, auriculate at the anterior lateral angles; proboscis entirely retractile within a transverse slit, below the short stout tentacles; eyes black, distinct, completely imbedded in and a little above the not perceptibly swollen bases of the tentacles, on their outer sides; verge situated behind the right tentacle, large, twisted, at first stout and subcylindrical, then deeply constricted; then compressed and expanded with a 1 conical papilla at the outer corner of the expansion much as in 0. corys Balch. -

1. in Tro Duc Tion
Cephalopods of the World 1 1. INTRO DUC TION Patrizia Jereb, Clyde F.E. Roper and Michael Vecchione he increasing exploitation of finfish resources, and the commercial status. For example, this work should be useful Tdepletion of a number of major fish stocks that formerly for the ever-expanding search for development and supported industrial-scale fisheries, forces continued utilization of ‘natural products’, pharmaceuticals, etc. attention to the once-called ‘unconventional marine resources’, which include numerous species of cephalopods. The catalogue is based primarily on information available in Cephalopod catches have increased steadily in the last 40 published literature. However, yet-to-be-published reports years, from about 1 million metric tonnes in 1970 to more than and working documents also have been used when 4 million metric tonnes in 2007 (FAO, 2009). This increase appropriate, especially from geographical areas where a confirms a potential development of the fishery predicted by large body of published information and data are lacking. G.L. Voss in 1973, in his first general review of the world’s We are particularly grateful to colleagues worldwide who cephalopod resources prepared for FAO. The rapid have supplied us with fisheries information, as well as expansion of cephalopod fisheries in the decade or so bibliographies of local cephalopod literature. following the publication of Voss’s review, meant that a more comprehensive and updated compilation was required, The fishery data reported herein are taken from the FAO particularly for cephalopod fishery biologists, zoologists and official database, now available on the Worldwide web: students. The FAO Species Catalogue, ‘Cephalopods of the FISHSTAT Plus 2009. -

Lab 5: Phylum Mollusca
Biology 18 Spring, 2008 Lab 5: Phylum Mollusca Objectives: Understand the taxonomic relationships and major features of mollusks Learn the external and internal anatomy of the clam and squid Understand the major advantages and limitations of the exoskeletons of mollusks in relation to the hydrostatic skeletons of worms and the endoskeletons of vertebrates, which you will examine later in the semester Textbook Reading: pp. 700-702, 1016, 1020 & 1021 (Figure 47.22), 943-944, 978-979, 1046 Introduction The phylum Mollusca consists of over 100,000 marine, freshwater, and terrestrial species. Most are familiar to you as food sources: oysters, clams, scallops, and yes, snails, squid and octopods. Some also serve as intermediate hosts for parasitic trematodes, and others (e.g., snails) can be major agricultural pests. Mollusks have many features in common with annelids and arthropods, such as bilateral symmetry, triploblasty, ventral nerve cords, and a coelom. Unlike annelids, mollusks (with one major exception) do not possess a closed circulatory system, but rather have an open circulatory system consisting of a heart and a few vessels that pump blood into coelomic cavities and sinuses (collectively termed the hemocoel). Other distinguishing features of mollusks are: z A large, muscular foot variously modified for locomotion, digging, attachment, and prey capture. z A mantle, a highly modified epidermis that covers and protects the soft body. In most species, the mantle also secretes a shell of calcium carbonate. z A visceral mass housing the internal organs. z A mantle cavity, the space between the mantle and viscera. Gills, when present, are suspended within this cavity. -

Double Function of Aptychi (Ammonoidea) As Jaw Elements and Opercula
Double function of aptychi (Ammonoidea) as jaw elements and opercula ULRICH LEHMANN AND CYPRIAN KULICKI Lehmann, U. & Kulicki, C. 1990 10 15: Double function of aptychi (Ammonoidea) as jaw elements and opercula. Lethaia, Vol. 23, pp. 325-331. Oslo. ISSN 0024-1164. Aptychi are calcitic coverings on the outer surface of organic ammonite lower jaws. They are similar in shape to that of the corresponding ammonite apertures. This observation and additional features of many aptychi are in harmony with their former interpretation as protective opercula. We suggest that they served as opercula in addition to functioning as jaws. The primary function of the lower jaws was thus secondarily extended to that of protective shields when they acquired their calcitic covering, while as lower jaws their importance dwindled to that of a more passive abutment. Phylogenetically, this seems to have started slowly in some anaptychi and became obvious with the first aptychi. OAmmonites, aptychus, operculum, jaw apparatus, evolution, function. Ulrich Lehmann, Geologisch-Palaontoiogisches Imtitut und Museum, Universitat Hamburg, Bundessrrape 55, 0-2000 Hamburg 13; Cyprian Kulicki, Polska Akademia Nauk. Zaklad Paleobiologii, Al. Zwirki i Wigury 93, P-02-089 Warszawa; 2nd January, 1990. The centuries-old discussion about the function normal position in life also, where they had been of anaptychi and aptychi entered a new phase embedded in a ventral mantle fold. From this when Lehmann (1970) demonstrated the anap- position, Trauth thought, they could be tilted tychi of Psiloceras, Pleuroceras and Arnioceras forward like a visor, when the animal withdrew to have been lower jaws. Soon afterwards, the into its body chamber.